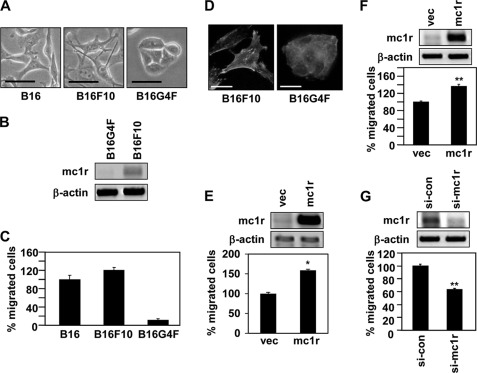
FIGURE 1.
MC1R expression levels affect melanoma cell migration. A, B16, B16F10 and B16G4F cells were distributed to tissue culture plates and incubated at 37 °C. After 48 h, digital photographs were taken using a phase contrast microscope. Scale bar, 20 μm. B, total RNA was extracted from exponentially growing B16G4F and B16F10 cells, and mRNA expressions were analyzed by RT-PCR, using β-actin as the loading control. C, Transwell migration assays were performed using FGF-2 (100 ng/ml) as a chemoattractant in the lower chamber. B16, B16F10, and B16G4F cells (5 × 104 cells/well) were allowed to migrate for 12 h, and migrated cells were stained with hematoxylin and eosin. The data shown are representative of three independent experiments. D, B16F10 cells (3.5 × 104 cells) or B16G4F cells (1.5 × 105 cells) were distributed to 12-well tissue culture plates and incubated for 48 h. The cells were fixed and immunostained with either anti-paxillin antibody (Texas Red) or phalloidin (FITC-conjugated). Scale bar, 20 μm. E, B16G4F cells were transfected with either vector (vec) or MC1R. Total RNA was extracted, and mRNA expressions were analyzed by RT-PCR. β-Actin was used as a control (top panel). Transwell migration assays were performed using FGF-2 (100 ng/ml) as a chemoattractant in the lower chamber. Transfected cells were allowed to migrate for 24 h, and migrated cells were stained with hematoxylin and eosin (bottom panel). The data shown are representative of three independent experiments. *, p < 0.01 versus vector. F, B16F10 cells were transfected with either vector or MC1R. After 24 h, total RNA was extracted, and mRNA expressions were analyzed by RT-PCR. β-Actin was used as a control (top panel). Migrated cells were analyzed by Transwell migration assay (bottom panel). G, B16F10 cells were transfected with siRNA targeting MC1R (si-mc1r). After 48 h, MC1R mRNA expression was analyzed by RT-PCR. β-Actin was used as a control (top panel). Migrated cells were analyzed by Transwell migration assay (bottom panel). si-con, control siRNA.