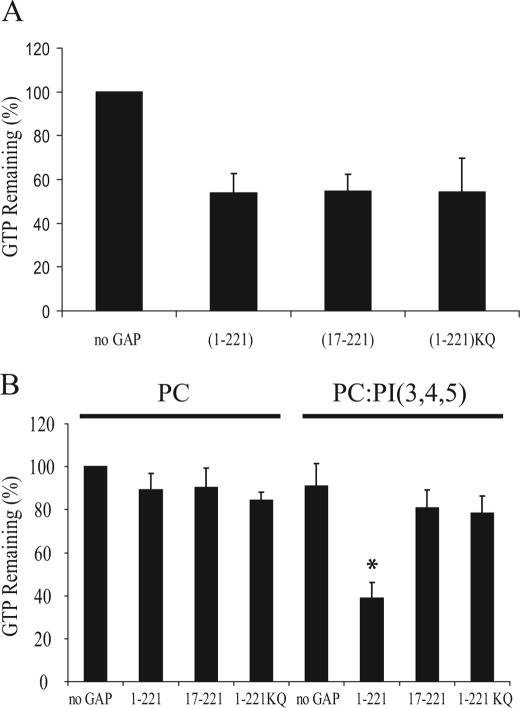
FIGURE 4.
The presence of PI(3,4,5)P3 on multilamellar vesicles loaded with prenylated Rac1 stimulates the GAP activity of CdGAP. A, [γ32P]GTP-loaded Rac1 was incubated with equal amounts of GST- CdGAP (1–221), CdGAP (17–221), or CdGAP (1–221)KQ. GTP hydrolysis was measured after 0 and 10 min incubation at 20 °C. The intrinsic Rac1 GTPase activity was set to 100% (no GAP). The graph shows a representative experiment performed in triplicate. Error bars, S.E. B, [γ32P]GTP-loaded geranylgeranylated Rac1 in the presence of MLVs was incubated with equal amounts of GST-CdGAP(1–221), CdGAP(17–221), or CdGAP(1–221)KQ. GTP hydrolysis was measured after 0 and 10 min incubation at 20 °C. The intrinsic Rac1 GTPase activity in the presence of control PC-containing MLVs was set to 100%. The graph shows a representative experiment performed in triplicate. Error bars, S.E. *, p < 0.001 for the comparison to the control (PC-containing MLVs).