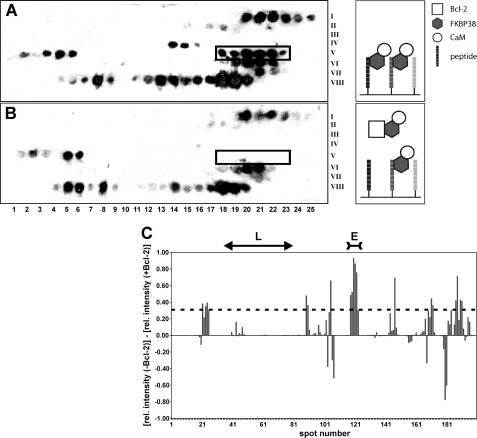
FIGURE 1.
A and B, Western blot analysis of Bcl-2 peptide library assays incubated with FKBP38 and holo-CaM in the absence and presence of Bcl-2(1–211), respectively. The membrane is partitioned into a matrix of eight rows (Roman numerals on the right), each consisting of 25 spots (Arabic numbers at the bottom). Every spot features a different decapeptide derived from the Bcl-2 sequence with a one-residue shift to the adjacent peptide spot. The spot number thus represents the Bcl-2 residue number of the first amino acid in each decapeptide. Black spots in A reveal a binding interaction of the respective decapeptide with FKBP38. Weaker spot intensities occur in B when Bcl-2 competed with the immobilized decapeptide for FKBP38. The spot range 118–123 (boxed) shows several contiguous spots with very pronounced intensity differences between A and B. The corresponding Bcl-2 segment emerged in subsequent tests as the only specific FKBP38-binding epitope. C, intensity bar graph showing the differences in the relative (rel.) chemiluminescence intensity between corresponding spots on the membrane without Bcl-2 (i.e. A) and on the membrane with Bcl-2 added (i.e. B). The effects observed in the spot range defining the flexible loop (L) region of Bcl-2 are negligible. The spot range defining the primary FKBP38-binding epitope (E) is also indicated at the top. The dashed line represents the mean + 1 S.D.