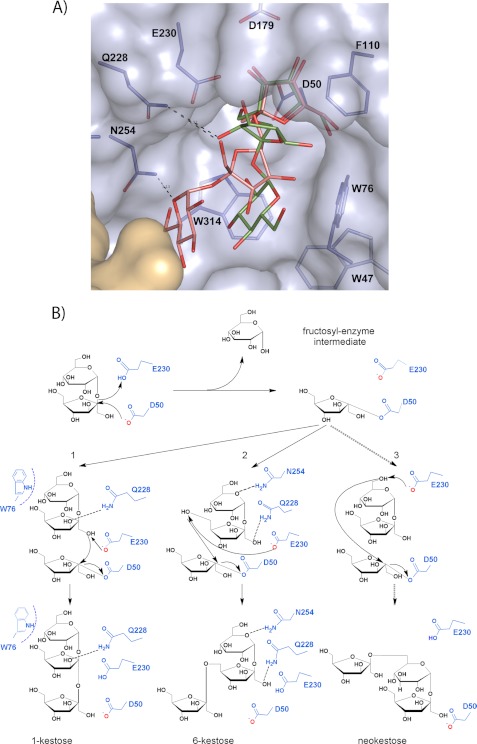
FIGURE 8.
Proposed mechanism of Ffase transfructosylating activity. A, the inferred position of the product 1-kestose (green) at the Ffase active site was obtained by superimposition of the C. intybus FEH 1-kestose coordinates (PDB code: 2AEZ) onto the fructose at the −1 subsite of the experimental Ffase-substrate complexes. The position of the product 6-kestose (pink) is inferred from superposition of its coordinates extracted from the CSD (Refcode CELGIC) onto the fructose found at subsite −1. The position of the Fru-Glu portion in both complexes represents a potential binding mode of acceptor sucrose for the formation of β(2–1) or β(2–6) linkage. B, schematic illustration of the proposed mechanism. The donor substrate sucrose is hydrolyzed by nucleophilic attack forming the fructosyl-Ffase intermediate. A subsequent sucrose molecule (the acceptor substrate) can enter the active site pocket with binding mode 1 generating 1-kestose or, alternatively, with binding mode 2 generating 6-kestose. Additionally, the Q228V/Q228T/Q228N and the N254T/N254D/N254A Ffase mutants are able to produce neokestose.