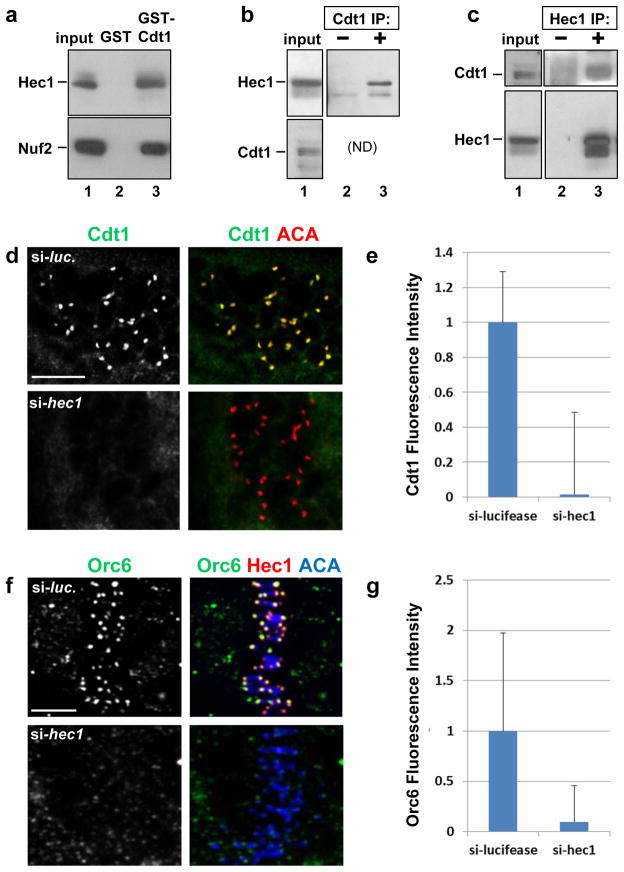
Figure 4. Hec1 is required for Cdt1 kinetochore localization.
(a) A lysate of T98G (human glioblastoma) cells was incubated with beads coated with bacterially-produced GST or GST-Cdt1, and the endogenous Hec1 and Nuf2 were detected in the input or bound fractions by immunoblotting. (b) Whole cell lysates of HeLa cells were incubated with pre-immune serum or anti-Cdt1 antibody, and endogenous Hec1 was detected in the input and immunecomplexes. (ND: Cdt1 co-migrates too closely to IgG heavy chain for detection in this IP.) (c) Whole cell lysates of HeLa cells were incubated with control serum or anti-Hec1 antibody; endogenous Cdt1 and Hec1 were detected in the input and immunecomplexes. (d) Nocodazole-treated PTK2 cells subjected to hec1 RNAi were fixed and stained with anti-Cdt1 antibody and anti-ACA antibody to mark kinetochores. (e) Quantification of Cdt1 kinetochore fluorescence intensity in d relative to control luciferase siRNA transfected cells; n = 80 kinetochores; p < 0.01. (f) HeLa cells were treated with control luciferase siRNA or hec1 siRNA, fixed and stained using anti-Orc6 antibody, anti-Hec1 antibody to monitor the Hec1 knockdown, and anti-ACA antibody to label kinetochores. (g) Quantification of Orc6 kinetochore fluorescence intensity in f relative to control luciferase siRNA transfected cells; n = 125 kinetochores; p < 0.01. Scale bars = 5 μm.