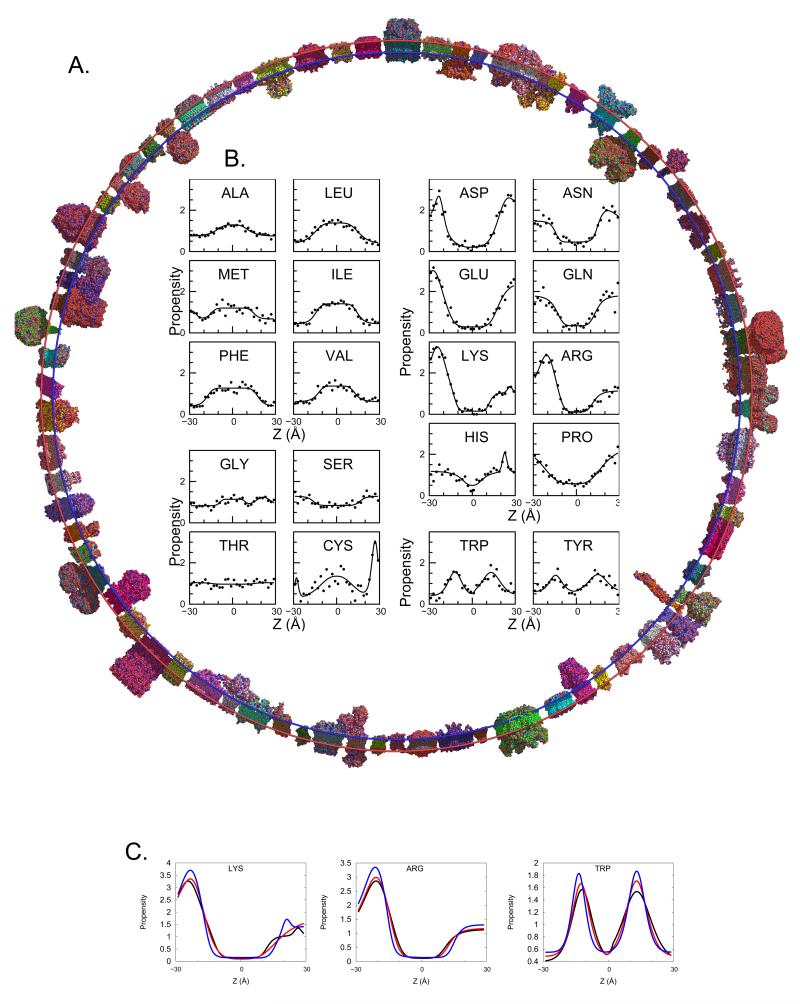
Figure 1.
Asymmetric Ez. The 76 protein database (A) is used to derive plots of amino acid propensity as a function of depth (Z) in the membrane. (B) Amino acid propensities (i.e. normalized frequencies, see Supplementary Methods) are grouped to hydrophobic residues, which prefer to be in the core of the bilayer; hydrophillic residues, which prefer to be in an aqueous environment; small, moderately polar residues; and aromatic residues, which prefer to partition in the head groups. (C) Comparison of the Cβ (black), Cγ (red), and functional group (blue) propensities for Lys, Arg, and Trp. Unlike hydrophobic residues in hydrophilic amino acids (Figure S1d) transitions get sharper as the atoms in question get further from the backbone. See also Figure S1 and Table S1.