Figure 4. In vivo and in vitro identification of Polo-like kinase 1 (Plk1) linear substrate motifs.
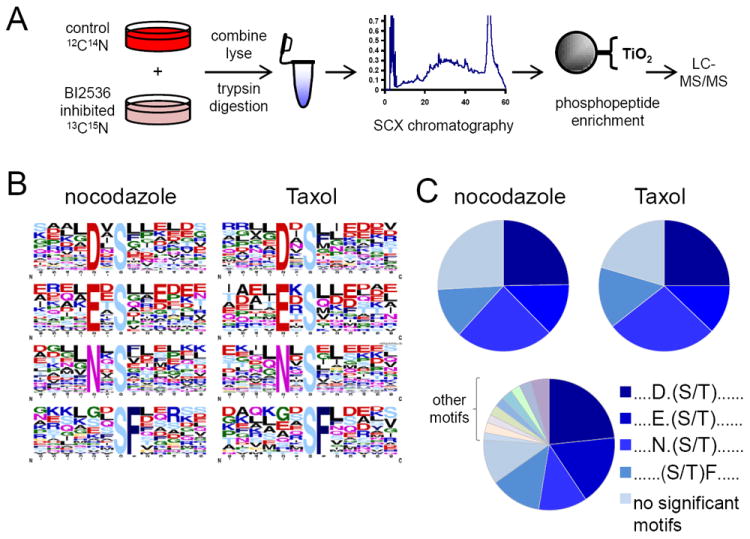
(A) SILAC-based workflow depicting in vivo identification of Plk1 substrates. HeLa cells were labeled with “heavy” and “light” amino acids in tissue culture, arrested in mitosis by nocodazole, differentially treated with a Plk1 inhibitor (BI-2536 dissolved in DMSO, heavy) or DMSO control (light), lysed, and trypsin-digested. Peptides were separated into 24 fractions by strong-cation exchange (SCX) chromatography and phosphopeptides were purified from non-phosphopeptides using titanium dioxide (TiO2) microspheres and analyzed by LC-MS/MS. Statistically significant motifs were extracted from phosphopeptides downregulated by 2.5-fold or more in the BI-2536-treated population by Motif-X. (B) Motif-X output for Plk substrates from nocodazole and Taxol-arrested HeLa cells. (C) Pie charts depicting the relative motif occurrence in HeLa cells arrested with nocodazole and Taxol, and in the in vitro kinase assay using purified Plk1 enzyme. Note that the relative distribution of primary Plk1 motifs observed in vivo with BI-2536 is similar to those with purified Plk1 in vitro. NS, no significant motifs. See also Figure S11 and Table S3.
