Abstract
Purpose
A retrospective review of consecutive adult patients undergoing scoliosis correction surgery was performed to compare the effects of aprotinin and tranexamic acid in blood conservation and to define a comprehensive blood conservation strategy for such surgery.
Methods
Medical records of all patients who underwent scoliosis correction surgery in this unit between January 2003 and December 2008 were reviewed. The patients were divided into three cohorts: group 1 receiving no antifibrinolytics, group 2 aprotinin and group 3 tranexamic acid. Information was collected regarding number of vertebral levels fused, pre- and post-operative haemoglobin, intra-operative blood loss and peri-operative autologous and allogenic blood transfusion performed.
Results
Aprotinin was used in 28 patients (38%), tranexamic acid in 26 (36%), while 19 (26%) received no antifibrinolytics. 21 patients had anterior surgery, 34 patients had posterior surgery and 18 had combined anterior and posterior procedures. Mean blood loss in the patients who received aprotinin and tranexamic acid was 710 and 738 ml, respectively. This was significantly less than the patients receiving no antifibrinolytics (972 ml, p = 0.037). Blood transfusion was required in only two patients undergoing anterior correction surgery.
Conclusion
Aprotinin and tranexamic acid reduce blood loss in adult spinal deformity correction surgery. With aprotinin being unavailable for clinical use, we recommend the use of tranexamic acid along with other blood conservation measures for adult spinal deformity correction surgery.
Keywords: Aprotinin, Tranexamic acid, Blood conservation, Adult deformity correction surgery
Introduction
Blood loss and blood conservation is an integral part of planning adult spinal deformity correction surgery. Blood loss is high because of the rich vascular network in the spine, wide exposure, duration of surgery and decortication osteotomies [1]. Increased blood loss leads to increased fibrinolysis, which in turn can lead to more blood loss. This can be a vicious cycle. The increased blood loss can lead to increases in mortality and morbidity [1].
Antifibrinolytic agents, such as aprotinin and tranexamic acid, have been shown to reduce blood loss in orthopaedic and other surgical procedures [2–11]. The primary aim of this study was to compare the effect of aprotinin and tranexamic acid in adult scoliosis correction surgery. A secondary purpose was to evaluate the blood conservation strategy used in this unit and to produce a protocol for blood cross matching for this surgical procedure.
Methodology
This retrospective study consisted of a review of 73 consecutive patients operated on for correction of spinal deformity in Cardiff and Vale NHS Trust Spinal Unit between January 2003 and December 2008. Patients were identified using the electronic database in the spinal unit and operating theatres in this hospital. All patients above 16 years of age were included in the study. Patients undergoing revision surgery were excluded. The patients were divided into three cohorts: group 1 receiving no antifibrinolytics, group 2 aprotinin and group 3 tranexamic acid. Information recorded from the case notes for each individual patient was as per Tables 1, 2 and 3. The data included number of vertebral levels fused, pre- and post-operative haemoglobin, intra-operative blood loss and peri-operative autologous and allogenic blood transfusion performed. During all cases a structured blood conservation strategy and a standard anaesthetic protocol was used.
Table 1.
Patient demographics
| No. of patients | 73 |
|---|---|
| Male:females | 21:52 |
| Age | 26.3 years (17–76) |
| BMI | 23.9 kg/m2 (18–29) |
Table 2.
Surgical details
| Approach | |
|---|---|
| Anterior | 21 |
| Posterior | 34 |
| Combined | 18 |
| Spinal levels fused | 10.8 (6–15) |
| Duration of surgery | 284 min (135–600) |
| Hospital stay | 9.1 days (4–15) |
Table 3.
Blood salvage and transfusion
| Use of cell salvage | 67 (91.7%) |
|---|---|
| Mean blood re-transfused (cell saver) | 578.7 ml |
| Pre-op Hb | 13.3 g/dl (10.4–17.4) |
| Post-op Hb | 10.4 g/dl (7.7–15.0) |
Outcome variables
In comparing the overall effects of antifibrinolytic agents during spinal correction surgery, we took into account both the efficacy and the safety of these agents. To compare the efficacy the blood loss and autologous or allogenic transfusion volumes were defined as the primary outcome measures. To compare the safety of the agents, complications including renal failure, myocardial infarction, stroke and post-operative serum creatinine and urea and were identified as the secondary outcome measures. This was based on previously reported complications with the use of aprotinin in cardiac surgery [12–16].
Statistical analysis
Statistical analysis was performed using the SPSS statistical program (Version 11; SPSS Inc., Chicago, IL). Student t test was used for parametric analysis and Mann–Whitney U test for non-parametric analysis in comparing aprotinin or tranexamic acid group individually with the group without antifibrinolytics. ANOVA was used to compare the three groups simultaneously. A p value of <0.05 was considered to be significant.
Comprehensive blood conservation strategy
Various blood conservation techniques have been described, usually in isolation. A comprehensive blood conservation strategy used in this unit is described.
Autologous transfusion is a recognised form of conserving blood during major spinal and orthopaedic surgeries. In this unit all patients undergoing major spinal deformity correction surgery have cell salvage technique employed (Cobe Brat 2®, Sorin group, Milano, Italia). Controlled hypotension plays a key role in blood conservation as does a meticulous surgical technique. During controlled hypotension, blood pressure is a poor guide to cardiac output, flow status and tissue perfusion. A low BP may be associated with both high and low output states that can be difficult to differentiate clinically. Young, healthy people can lose up to 40% of circulating blood volume before BP falls due to compensatory mechanisms.
Central venous pressure (CVP) has long been used as a guide to intravascular volume. However, it is not a good indicator either of atrial and ventricular filling pressures or of blood loss. Due to redistribution of blood within the circulation peripheral vasoconstriction, there may be considerable blood loss before there is a change in CVP. It also does not reflect changes in vascular compliance or of myocardial contractility. It may be a better guide if used as a dynamic indicator when coupled with intravenous volume challenges, but this is difficult to interpret in the intra-operative setting. The reliability of CVP with the patient in prone position is also questionable [17].
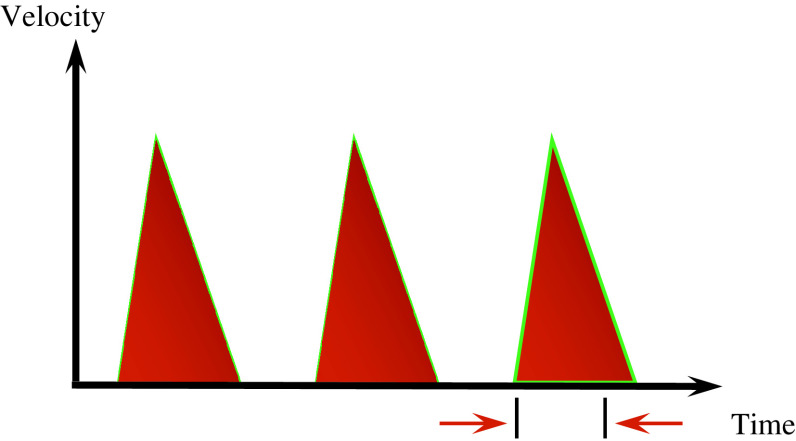
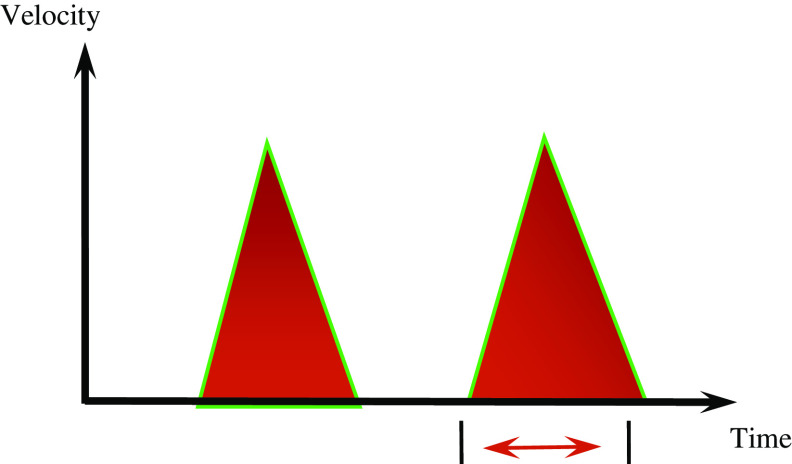
Oesophageal Doppler is a reliable, non-invasive and technically easy way of monitoring cardiac output and intravascular fluid status [17]. In this unit, we use the CardioQ (CardioQ™ Deltex Medical, Sussex, UK) oesophageal monitor. A Doppler probe is placed in the oesophagus with the wave form directed posterior to the aorta. The monitor detects the velocity of the blood flow in the descending aorta and this is depicted as velocity over time waveforms. With fluid optimisation a narrow waveform base changes to a wide waveform base (Figs. 1, 2) and there is increased flow time corrected (for heart rate) (FTc). Typically the FTc for normovolemia is between 0.330 and 0.360 s.
Fig. 1.
Velocity over time waveform graph obtained with trans-oesophageal Doppler. With hypovolemia, there is narrow waveform base and reduced flow time
Fig. 2.
Velocity over time waveform graph obtained following fluid optimisation. There is widened waveform base and increase in flow time
Fibrinolytics were used only if there were no contraindications. This included deranged renal function with elevated serum creatinine. Aprotinin was administered intravenously as 1 × 106 KIU at induction followed by an infusion of 0.5 × 106 KIU/h during the surgery and for 2 h post-operatively. Tranexamic acid, 2 gm, was administered intravenously at induction.
Results
Patient demographics and the surgical details are as per Tables 1 and 2, respectively. Cell salvage was used in 67 patients (Table 3). In the remaining six patients, as the surgery was anterior short segment fusion in patients without any other co morbidities, cell salvage was not used.
Twenty-eight patients (38.3%) received aprotinin, 26 (35.6%) received tranexamic acid and 19 (26.0%) received no antifibrinolytics. Of the study group, seven patients were operated before we started to use antifibrinolytic agents. Two patients had contraindications to the use of aprotinin and received no antifibrinolytics. Ten patients received no antifibrinolytics because the segmental fusion was short and anti-fibronlytic therapy deemed inappropriate. Since the withdrawal of aprotinin from clinical use due to safety concerns, all patients undergoing spinal deformity correction surgery receive tranexamic acid in this unit unless contraindicated.
Four patients received intra-operative blood transfusion and another 15 received post-operative blood transfusion. Blood loss and the transfusions preformed are further elaborated in Tables 4 and 5. Of the 21 patients who underwent anterior surgery only 2 required post-operative blood transfusion. No patient in this group required intra-operative transfusion. No patient in any group was found to have any renal complication as indicated by raised post-operative serum creatinine. No cases of peri-operative myocardial infraction, stroke or encephalopathy were identified. One patient in the aprotinin group had post-operative DVT. This was not statistically significant (p = 0.071).
Table 4.
Results comparing the three groups (aprotinin, tranexamic acid and no fibrinolytics)
| Aprotinin | Tranexamic acid | No fibrinolytics | |
|---|---|---|---|
| Number of patients | 28 | 26 | 19 |
| Sex (M:F) | 9:19 | 5:21 | 7:12 |
| Age (mean) | 24.8 (17–68) | 26.1 (19–74) | 28.8 (21–76) |
| BMI (mean) | 23.5 (18–28) | 23.9 (19–28) | 22.6 (19–29) |
| Levels fused | 12.3 (7–15) | 11.9 (8–15) | 7.1 (6–11) |
| Duration (min) | 298 (135–600) | 312 (186–480) | 225 (178–386) |
| Indication | |||
| Idiopathic scoliosis | 26 | 24 | 18 |
| Degenerative | 1 | 2 | 1 |
| Inflammatory arthropathy | 1 | 0 | 0 |
| Mean blood loss | 710 ml (73%) | 738 ml (76%) | 972 ml |
| Blood loss as percentage of expected blood volume | 16.7% | 17.5% | 21.1% |
| Mean blood loss per level fused | 57.7 ml | 62.0 ml | 136.9 ml |
| Cell saver (mean autologous transfusion) | 425 ml | 457 ml | 665 ml |
| Allogenic transfusion (number of patients) | 6 | 7 | 6 |
| Approach | |||
| Anterior | 7 | 6 | 8 |
| Posterior | 11 | 12 | 11 |
| Combined | 10 | 8 | 0 |
Table 5.
Anterior approach and blood transfusion in the three groups
| Aprotinin | Tranexamic acid | No fibrinolytics | |
|---|---|---|---|
| Number of patients | 7 | 6 | 8 |
| Sex (M:F) | 3:4 | 2:4 | 3:5 |
| Age (mean) | 27.1 | 29.3 | 26.8 |
| BMI (mean) | 20.1 | 21.8 | 21.4 |
| Levels fused | 7.1 | 7.0 | 6.8 |
| Duration (min) | 247 | 261 | 284 |
| Mean blood loss (ml) | 310 | 344 | 472 |
| Cell saver (autologous transfusion) (ml) | 305 | 331 | 438 |
| Allogenic transfusion (number of patients/units) | 0 | 1 | 1 |
The mean intra-operative blood loss in the group without any antifibrinolytics was 972 ml, which reduced to 710 and 738 ml, respectively, in the groups using aprotinin and tranexamic acid. The reduction was statistically significant (p = 0.037). Hence, there was 27% reduction with aprotinin and 24% with tranexamic acid. Mean intra-operative blood loss per segment fused was 57.7, 62.01 and 136.9 ml with the use of aprotinin, tranexamic acid and no antifibrinolytics, respectively (p = 0.016). Similarly, for the anterior approach intra-operative blood loss per segment fused was 43.7 ml for aprotinin, 49.1 ml for tranexamic acid and 69.4 ml without using any antifibrinolytics. This difference was statistically significant (p = 0.041). As shown in Table 4, blood loss for each group was also calculated as a percentage of the expected blood volume, which in turn was calculated using Nadler’s method [18]. This enables to normalise the height and weight of the patients for the blood loss. The difference was statistically significant (p = 0.043).
Discussion
Adult spinal deformity correction surgery can be associated with major blood loss with requirement for blood transfusion. Blood loss depends on the number of levels fused, duration of surgery, and physical status of the patient [1]. Concerns regarding the safety of transfused blood have led to the development of a range of interventions to minimise blood loss during such major surgery. Hence, a comprehensive blood conservation strategy gains paramount importance to ensure decreased complications in the peri-operative period and the overall success of the operative treatment.
Aprotinin inhibits several serine proteases, specifically trypsin, chymotrypsin and plasmin and kallikrein [19]. Its action on kallikrein leads to the inhibition of the formation of factor XIIa. As a result, both the intrinsic pathway of coagulation and fibrinolysis are inhibited. Its action on plasmin independently slows fibrinolysis [20]. Tranexamic acid is a synthetic derivative of the amino acid lysine that exerts its antifibrinolytic effect through the reversible blockade of lysine binding sites on plasminogen molecules [4] and by decreasing induced fibrinolysis [8].
Various antifibrinolytic agents have been used with success in cardiac surgery [2, 3], obstetrics [4], upper gastrointestinal haemorrhage [4], liver transplant [5], and other major orthopaedics surgeries with potential for increased blood loss [6–8]. However, with regards to the efficacy of these agents, there is evidence to the contrary as well. In patients undergoing orthotopic liver transplantation there was no effective reduction in blood loss or transfusion requirement with tranexamic acid and aprotinin administration [21]. A meta-analysis concluded aprotinin to be effective only in revision total hip arthroplasties rather than primary hip replacements. It also found efficacy of tranexamic acid to reduce blood loss and blood transfusion in hip arthroplasty to be inconclusive [22].
Most of the studies evaluating anti fibrinogen agents in scoliosis correction surgery are on paediatric and adolescent patient groups. In prospective randomized controlled studies aprotinin [9, 10] and tranexamic acid [10, 11] have been found to significantly decrease blood loss and transfusion requirements in paediatric patients undergoing scoliosis correction surgery. Sethna et al. [23] found 41% reduction in blood loss in patients receiving tranexamic acid in comparison to placebo in a prospective study on paediatric scoliosis patients.
Spinal deformity in adults can include stiffer curves in comparison to the paediatric patients. The surgery can be more complex and involve longer surgical time, all leading to increased potential for blood loss. In addition, adult and elderly patients undergoing spinal surgery potentially have more co-morbidities. This can also make any intra-operative blood loss less well tolerated. Establishing the safety of the antifibrinolytic agents in the adult patients is therefore of much importance.
In a retrospective study on adult spinal deformity surgeries, Tayyab et al. [24] concluded aprotinin to be safe and effective in adult spinal deformity correction surgery. The study found 43% reduction in autologous blood transfusion and 46% reduction in allogenic blood transfusion with the use of aprotinin. However, tranexamic acid was not evaluated in that study [24]. Kasimian et al. [25] found aprotinin to be safe and cost effective for patients with neuromuscular scoliosis. They found reduction in intra-operative blood loss and post-operative blood transfusion requirements.
Patient population in the three groups was comparable in age, BMI and sex distribution in this study. Antifibrinolytics were preferentially used in surgery involving long segments. This was reflected in the mean number of vertebral levels fused in the three groups (Table 3). Levels fused were more in the groups using antifibrinolytics. There was significant difference in blood loss in the groups receiving aprotinin and tranexamic acid in comparison to the patient group receiving no antifibrinolytics (p = 0.037). The difference was more significant if the number of segments fused was taken into consideration. The mean blood loss per segment was 57.7, 62.01 and 136.9 ml with the use of aprotinin, tranexamic acid and no antifibrinolytics, respectively (p = 0.026). 10 patients received no antifibrinolytics as this was deemed unnecessary for the procedure. However, this group lost more blood than the other two despite involving a smaller curve. Tranexamic acid had lesser reduction in the blood loss as compared to aprotinin. However, the difference between the two groups was not statistically significant (p = 0.085). The drug reduces post-operative blood losses and transfusion requirements, with potential cost and tolerability advantages over aprotinin [4].
Concerns had been raised about the safety of aprotinin after an association between increased renal dysfunction [12, 13, 15, 16], myocardial infarction, encephalopathy, stroke [16] and mortality [12, 14, 16] was shown in retrospective observational studies. The risk of acute renal failure is higher in women over the age of 60 [13]. An increase in all-cause mortality with aprotinin relative to tranexamic acid or epsilon-aminocaproic acid was seen in a pre-planned periodic analysis of the large Blood conservation using Antifibrinolytics in a Randomized Trial study. The latter finding resulted in the trial being halted, and aprotinin has subsequently been withdrawn from the market pending detailed analysis of efficacy and safety results from the study [12].
No severe complications have not been reported for tranexamic acid in current literature. However, most of the studies are limited by a small sample size and hence complications can be missed. We had no thrombo-embolic or renal dysfunction complications in our series but still recommend use of adequate peri-operative investigations and observations especially in high risk patients with other co-morbidities. The expected incidence of thrombo-embolism is small, hence a prospective study with larger number of patients would be needed to identify any association with some conformity.
Blood transfusion is a costly transplantation of a tissue and puts the health of the recipient at risk. Blood transfusions are common after major orthopaedic and spinal surgery and the total saving potential is substantial if the blood loss could be reduced [26].
Using a comprehensive blood conservation strategy and based upon the above results, this unit has evolved a more defined protocol for blood transfusion. Patients undergoing only anterior surgery have only ‘group and save’ performed rather than ‘crossmatch’ pre-operatively.
This was a retrospective study with unequal and less number of patients in each group. The authors acknowledge that there is a potential bias in the study as most of the cases without antifibrinolytics were performed early on in the series. This could influence the results due to the team’s learning curve with the surgical procedure and the overall blood conservation strategy.
Conclusion
Aprotinin and tranexamic acid significantly reduce blood loss during adult spinal deformity correction surgery. With aprotinin no longer available, tranexamic acid may be used effectively. This study has identified no complications with the use of tranexamic acid. A comprehensive blood conservation strategy reduces homologous blood transfusion. Cross matching is not necessary for anterior surgery alone.
Conflict of interest
The authors have no conflicting interests.
References
- 1.Szpalski M, Weiskopf RB, Gunzburg R, Aebi M. Blood loss in adult spinal surgery. Haemostasis in spine surgery, Part 2. Berlin: Springer; 2005. pp. 3–5. [Google Scholar]
- 2.Bulutcu FS, Ozbek U, Polat B, Yalçin Y, Karaci AR, Bayindir O. Which may be effective to reduce blood loss after cardiac operations in cyanotic children: tranexamic acid, aprotinin or a combination? Paediatr Anaesth. 2005;15:41–46. doi: 10.1111/j.1460-9592.2004.01366.x. [DOI] [PubMed] [Google Scholar]
- 3.Santos AT, Kalil RA, Bauemann C, Pereira JB, Nesralla IA. A randomized, double-blind, and placebo-controlled study with tranexamic acid of bleeding and fibrinolytic activity after primary coronary artery bypass grafting. Braz J Med Biol Res. 2006;39:63–69. doi: 10.1590/s0100-879x2006000100007. [DOI] [PubMed] [Google Scholar]
- 4.Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57:1005–1032. doi: 10.2165/00003495-199957060-00017. [DOI] [PubMed] [Google Scholar]
- 5.Ickx BE, van der Linden PJ, Melot C, Wijns W, de Pauw L, Vandestadt J, Hut F, Pradier O. Comparison of the effects of aprotinin and tranexamic acid on blood loss and red blood cell transfusion requirements during the late stages of liver transplantation. Transfusion. 2006;46:595–605. doi: 10.1111/j.1537-2995.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 6.Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003;31:529–537. doi: 10.1177/0310057X0303100507. [DOI] [PubMed] [Google Scholar]
- 7.Zufferey P, Merquiol F, Laporte S, Decousus H, Mismetti P, Auboyer C, Samama CM, Molliex S. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105:1034–1046. doi: 10.1097/00000542-200611000-00026. [DOI] [PubMed] [Google Scholar]
- 8.Ekbäck G, Axelsson K, Ryttberg L, Edlund B, Kjellberg J, Weckström J, Carlsson O, Schött U. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91:1124–1130. doi: 10.1213/00000539-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Cole JW, Murray DJ, Snider RJ, Bassett GS, Bridwell KH, Lenke LG. Aprotinin reduces blood loss during spinal surgery in children. Spine. 2003;28:2482–2485. doi: 10.1097/01.BRS.0000090835.45437.7F. [DOI] [PubMed] [Google Scholar]
- 10.Bitan FD. Aprotinin in spine surgery: review of the literature. Orthopedics. 2004;27:s681–s683. doi: 10.3928/0147-7447-20040602-10. [DOI] [PubMed] [Google Scholar]
- 11.Neilipovitz DT, Murto K, Hall L, Barrowman NJ, Splinter WM. A randomized trial of tranexamic acid to reduce blood transfusion for scoliosis surgery. Anesth Analg. 2001;93:82–87. doi: 10.1097/00000539-200107000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Neilipovitz DT. Tranexamic acid for major spinal surgery. Eur Spine J. 2004;13:S62–S65. doi: 10.1007/s00586-004-0716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okubadejo GO, Bridwell KH, Lenke LG, Buchowski JM, Fang DD, Baldus CR, Nielsen CH, Lee CC (2007) Aprotinin may decrease blood loss in complex adult spinal deformity surgery, but it may also increase the risk of acute renal failure. Spine 15(32):2265–2271 [DOI] [PubMed]
- 14.Fergusson DA, Hébert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, Teoh K, Duke PC, Arellano R, Blajchman MA, Bussières JS, Côté D, Karski J, Martineau R, Robblee JA, Rodger M, Wells G, Clinch J, Pretorius R, BART Investigators A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;358:2319–2331. doi: 10.1056/NEJMoa0802395. [DOI] [PubMed] [Google Scholar]
- 15.Brown JR, Birkmeyer NJ, O’Connor GT. Meta-analysis comparing the effectiveness and adverse outcomes of antifibrinolytic agents in cardiac surgery. Circulation. 2007;115:2801–2813. doi: 10.1161/CIRCULATIONAHA.106.671222. [DOI] [PubMed] [Google Scholar]
- 16.Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–365. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 17.Soliman DE, Maslow AD, Bokesch PM, Strafford M, Karlin L, Rhodes J, Marx GR. Transoesophageal echocardiography during scoliosis repair: comparison with CVP monitoring. Can J Anaesth. 1998;45:925–932. doi: 10.1007/BF03012298. [DOI] [PubMed] [Google Scholar]
- 18.Nadler SB, Hidalgo JU, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224–232. [PubMed] [Google Scholar]
- 19.Mahdy AM, Webster NR. Perioperative systemic haemostatic agents. Br J Anaesth. 2004;93:842–858. doi: 10.1093/bja/aeh227. [DOI] [PubMed] [Google Scholar]
- 20.Mannucci PM. Hemostatic drugs. N Engl J Med. 1998;339:245–253. doi: 10.1056/NEJM199807233390407. [DOI] [PubMed] [Google Scholar]
- 21.Dalmau A, Sabaté A, Koo M, Bartolomé C, Rafecas A, Figueras J, Jaurrieta E. The prophylactic use of tranexamic acid and aprotinin in orthotopic liver transplantation: a comparative study. Liver Transpl. 2004;10:279–284. doi: 10.1002/lt.20075. [DOI] [PubMed] [Google Scholar]
- 22.Gill JB, Rosenstein A. The use of antifibrinolytic agents in total hip arthroplasty: a meta-analysis. J Arthroplasty. 2006;21:869–873. doi: 10.1016/j.arth.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Sethna NF, Zurakowski D, Brustowicz RM, Bacsik J, Sullivan LJ, Shapiro F. Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery. Anesthesiology. 2005;102:727–732. doi: 10.1097/00000542-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Tayyab NA, Mariller MM, Rivlin M, Berekashvili K, Bitan FD, Casden AM, Kuflik P, Neuwirth MG. Efficacy of Aprotinin as a blood conservation technique for adult deformity spinal surgery: a retrospective study. Spine. 2008;33:1775–1781. doi: 10.1097/BRS.0b013e31817b87c4. [DOI] [PubMed] [Google Scholar]
- 25.Kasimian S, Skaggs DL, Sankar WN, Farlo J, Goodarzi M, Tolo VT. Aprotinin in pediatric neuromuscular scoliosis surgery. Eur Spine J. 2008;12:1671–1675. doi: 10.1007/s00586-008-0790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty aves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76:314–319. [PubMed] [Google Scholar]