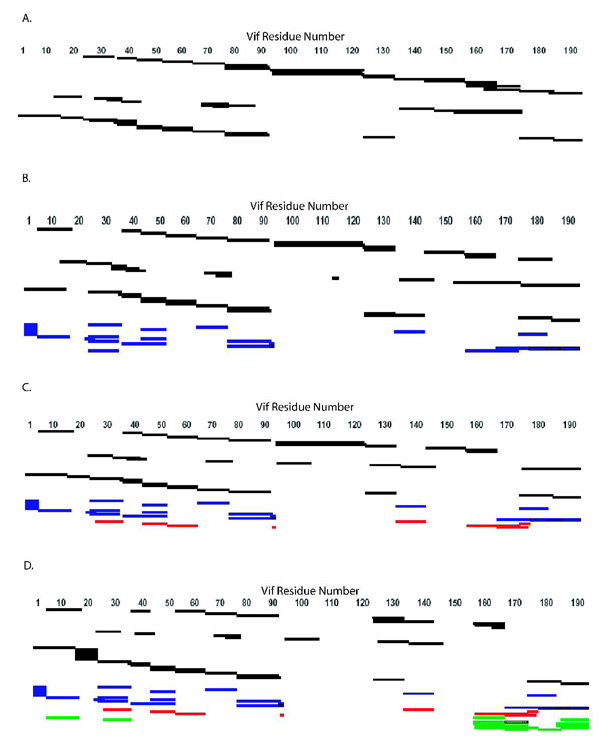
Figure 3. Peptide Sequence Coverage Map from MALDI-TOF, LC-ion trap-MS, and LC-QTof-MS Data.
Coverage maps of tryptic and chymotryptic peptides identified from MALDI-TOF, LC-ion trap-MS, and LC-QTof-MS, and tryptic cross-links identified from MALDI-TOF. Peptides and cross-links were identified via comparison of the experimental molecular weight with a list of theoretical molecular weights calculated from ProteinProspector or GPMAW as well as by a corresponding ion 4Da larger for a peptide and 8Da larger for a cross-link. (A) Noncross-linked. (B) Monomer. (C) Dimer. (D) Trimer. Identified regions are those that are cross-linked to each other and those that are protected. Black: peptides; blue: intramolecular cross-links; red: cross-links in the dimer and trimer; green: trimer cross-links.