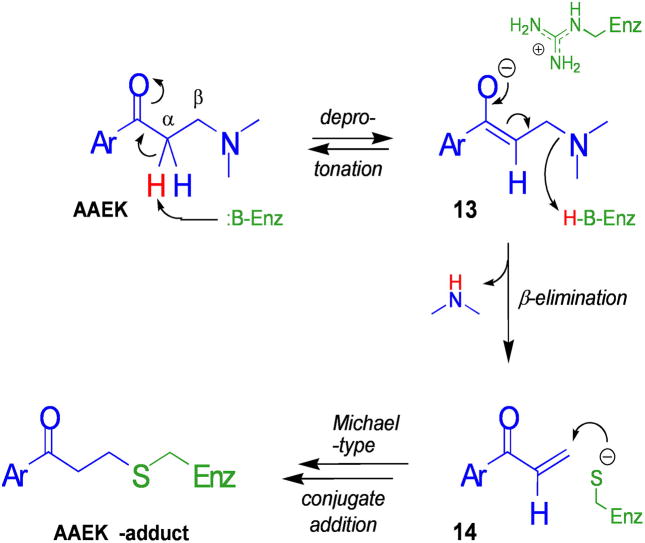
FIGURE 7. Model for the mechanism of inhibition of sortase by AAEKs.
Deprotonation of the α carbon is conjectured to occur via a base in the active site of sortase. This generates enolate 13, which may be stabilized in a manner similar to the oxyanion intermediate of sortase during catalysis (e.g. by the guanidinium of a conserved active site arginine). This intermediate eliminates an amine, here dimethylamine, from the β-position to generate 14. The elec-trophilic nature of 14 allows it to serve as an acceptor in a Michael-type conjugate addition by the thiol of the active site cysteine. This resulting enolate might also be stabilized by the guanidinium moiety; subsequent protonation by enzyme or medium would then generate the stable AAEK thioether adduct observed.