Abstract
We conducted a serologic survey of four high-priority pig-associated viral zoonoses, Japanese encephalitis virus (JEV), hepatitis E virus (HEV), Nipah virus (NiV), and swine influenza virus (SIV), in Laos. We collected blood from pigs at slaughter during May 2008–January 2009 in four northern provinces. Japanese encephalitis virus hemagglutination inhibition seroprevalence was 74.7% (95% confidence interval [CI] = 71.5–77.9%), JEV IgM seroprevalence was 2.3% (95% CI = 1.2–3.2%), and HEV seroprevalence was 21.1% (95% CI = 18.1–24.0%). Antibodies to SIV were detected in 1.8% (95% CI = 0.8–2.8%) of pigs by screening enzyme-linked immunosorbent assay, and only subtype H3N2 was detected by hemagglutination inhibition in two animals with an inconclusive enzyme-linked immunosorbent assay result. No NiV antibody–positive pigs were detected. Our evidence indicates that peak JEV and HEV transmission coincides with the start of the monsoonal wet season and poses the greatest risk for human infection.
Introduction
Pig associated viral zoonoses pose a significant threat to human populations in Southeast Asia. Of particular importance are the encephalitic Japanese encephalitis virus (JEV) and Nipah virus (NiV), the pathogenic strains of swine influenza virus (SIV), and zoonotic genotypes of hepatitis E virus (HEV). All have been reported in Southeast Asia and pigs have been directly linked to human disease.1–4
Japanese encephalitis virus is a major cause of death and disability in Asia and is transmitted by paddy-breeding Culex mosquitoes, primarily Culex tritaeniorhynchus, in a zoonotic cycle involving ardeid wading birds (herons and egrets), pigs, and humans.5 During times of peak transmission, amplification of JEV in immunologically naive pigs within the vicinity of human habitation precedes epidemic transmission to humans.5 Pig production industries are also affected by JEV infection and economic losses occur through decreased productivity associated with reproductive failure.6 In Laos, a significant proportion of the human population live within the vicinity of rice paddies,7 and pig production is practiced in rural, peri-urban, and urban environs8,9 ensuring suitable conditions for JEV transmission from pigs to humans.
Hepatitis E virus is primarily a water-borne virus generally causing a self-limiting acute hepatitis in humans, but is noted for causing fulminant hepatitis and a high case-fatality rate during pregnancy in certain environments.10,11 Consumption of contaminated meat and occupational exposure are also recognized as important modes of zoonotic transmission.10,11 Four distinct genotypes of HEV have been characterized, but only genotypes 3 and 4 are considered zoonotic and pigs are recognized as an especially important source of human infection.12 Previous studies in Laos indicate that 16–18% of the human population13,14 and up to 50% of the pig population15 have serologic evidence of exposure to HEV. Zoonotic HEV is emerging as the dominant form of human HEV disease in eastern and southern China16,17 and clearly demonstrates the risks posed to the human population of Laos.
Nipah virus is an emerging encephalitic virus that has been associated with significant outbreaks of human disease in Malaysia, Singapore, India, and Bangladesh.18–21 The natural reservoir hosts of NiV are Pteropid fruit bats (flying foxes), which can serve as the source of infection for a range of susceptible mammals, including humans and pigs.22 The known geographic range of the virus has extended over recent years with serologic and molecular evidence demonstrating NiV to be present in fruit bat species in Cambodia, Indonesia, and Thailand.23–26 Pigs can be a critical intermediary host, acting as a bridge in transmission between infected wild fruit bats and humans. This finding was demonstrated in outbreaks in Malaysia and Singapore in which human NiV disease was associated with exposure to infected pigs or pig carcasses.2 However, food-borne and human-to-human transmission has also been demonstrated in South Asia.27,28 Pteropid bats are prevalent in Laos, but are currently threatened by habitat loss and hunting.29 This incursion into the natural fruit bat habitat possibly exposes human and pig populations to NiV infection and an investigation of infection in pigs is required.
The prevalence of SIV in pig populations worldwide can vary greatly, and a number of HN subtypes may circulate in any particular country. Five subtypes (H1, H3, H5, H7, and H9) in China30 and three subtypes (H1N1, H1N2, and H3N2) in Thailand31 have been reported in their respective pig populations. Pigs have a role in the emergence of novel pathogenic strains through the mixing and re-assortment of human-, avian-, and swine-adapted influenza viruses.32 In light of the highly pathogenic avian influenza virus epidemic in Laos33 and the emergence of the pandemic H1N1 influenza virus in 2009, SIV warrants investigation in Laos.
There remains a scarcity of good-quality data related to the role of pigs as a reservoir of pathogens causing human disease in Laos. The present study was conducted within the scope of a broader pig zoonoses project and aimed to determine the serologic prevalence of these four important viral zoonoses and to assess if age, breed, temporal, and spatial factors were associated with serologic evidence of infection.
Materials and Methods
Ethics statement.
The research protocols were reviewed and approved by the Murdoch University Animal Ethics Committee (project no. R2108/07), which adheres to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. The Lao Department of Livestock and Fisheries does not, at this time, have a committee to review and approve scientific research protocols involving animals.
Study sites and survey design.
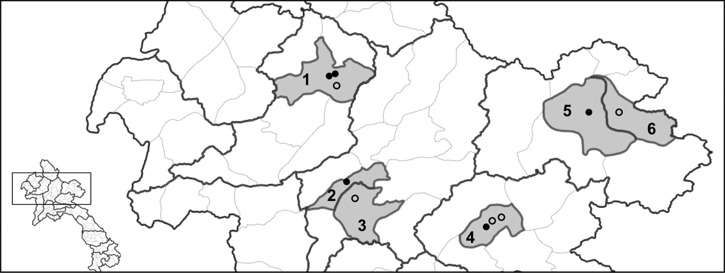
The surveys were conducted at three slaughter points each in Xiengkhuang and Oudomxay Provinces during May–September 2008 and at two collection points each in Huaphan and Luangprabang Provinces during October 2008–January 2009 (Figure 1). The survey team consisted of trained district and provincial agricultural and forestry government staff who visited the slaughter points approximately every two weeks. A blood sample was obtained from all pigs brought for slaughter on the nights the survey team visited slaughter sites. Blood samples were centrifuged, and the serum fraction was removed and stored at –20°C before testing. An aliquot of each serum sample was sent to the Armed Forces Research Institute for Medical Sciences (AFRIMS) (Bangkok, Thailand) for JEV and HEV testing and to the Commonwealth Scientific and Industrial Research Organisation (CSIRO)Australian Animal Health Laboratory (Geelong, Victoria, Australia) for SIV and NiV testing.
Figure 1.
Study sites in northern Laos. 1 = Xay District, Oudomxay Province; 2 = Luangprabang District, Luangprabang Province; 3 = Xiengngeun District, Luangprabang Province; 4 = Pek District, Xiengkhuang Province; 5 = Xamneua District, Huaphan Province; 6 = Viengxay District, Huaphan Province. Black dots indicate abattoirs or slaughterhouses (> 20 pigs per night) and black circles indicate slaughter points (< 5 pigs per night). The black dot in Luangprabang District (2) represents an amalgamation of home slaughter points caused by reconstruction of abattoir at the time of survey.
Laboratory techniques.
Japanese encephalitis virus hemagglutination inhibition assay.
Serum samples were pre-treated with acetone, and the ability of test serum antibodies to inhibit JEV sucrose-acetone–extracted mouse brain antigens agglutinating goose erythrocytes was assayed by using a microtiter adaptation of the method of Clarke and Casals34 with an initial dilution of 1:10. Serum samples were serially diluted to 1:80 and hemagglutination inhibition (HI) titers > 10 were considered positive for JEV-specific antibody.35
Japanese encephalitis virus IgM enzyme-linked immunosorbent assay.
The AFRIMS in-house JEV IgM enzyme-linked immunosorbent assay (ELISA) for pig serum,35 adapted from human JEV IgM immunoassays,36–38 was used to test pigs for evidence of an active or recent infection at the time the survey was conducted. In brief, IgM in the test sample was captured out of solution onto the solid phase of polystyrene plate wells previously coated with goat anti-swine IgM. A 1:100 dilution of test serum was added to the wells. Sucrose- and acetone-extracted suckling mouse brain JEV antigen was added to each well, and peroxidase-conjugated human anti-flavivirus-hyperimmune IgG was added to label the bound antigen. Color developed by the addition of o-phenylenediamine substrate, and color development was stopped by the addition of 4 M H2SO4. Absorbance at 492 nm (A492) was read spectrophotometrically on a microplate reader (Spectra Max 340 PC; Molecular Devices, Inc., Sunnyvale, CA). Negative (NS, A492 < 0.1) and positive standards (WPC, A492 0.4 + 0.15) were added to each test plate. Assay results were expressed as units, which were calculated by using the formula units = 100 × (A492 test sample – A492 NS)/(A492 WPC – A492 NS), and 40 units of antibody defines the lower limit of positivity.35
Hepatitis E virus serologic analysis.
Detection of HEV IgG was modified from an ELISA reported by Innis and others39 and Wang and others.40 In brief, 96-well plates were coated with 100 μL of recombinant capsid protein antigen of HEV open reading frame 2/3 (GenWay Biotech, Inc., San Diego, CA) diluted 1:5,000 in 0.05 M carbonate-bicarbonate buffer, pH 9.6 (Sigma-Aldrich, St. Louis, MO). The plate was incubated at 37°C for 4 hours and then overnight at 4°C. The coating buffer was discarded and the plate was machine washed (SkanWasher; Skatron, Sterling, VA) with wash buffer (0.5% Tween 20 in phosphate-buffered saline) (Sigma-Aldrich). Three hundred microliters of blocking buffer (0.5% casein, 0.5% bovine serum albumin in phosphate-buffered saline, pH 7.4) was added to each well and incubated for 1 hour at 37°C. Plates were then machine-washed. One hundred microliters of two negative control, three positive control, and each pig serum diluted 1:500 in blocking buffer was added in duplicate wells and incubated at 37°C for 1 hour. Horseradish peroxidase–conjugated goat anti-swine IgG (Kirkegaard and Perry Laboratories, Gaithersburg, MD) diluted 1:4,000 in blocking buffer containing 0.2% Tween 20 was added to each well. The plate was incubated at 37°C for 30 minutes and then machine-washed. Samples were visualized with the addition of SureBlue (3,3′,5,5′-tetramethylbenzidine) substrate (Kirkegaard and Perry Laboratories) and after incubation at room temperature for 10 minutes, the reaction was stopped by the addition of 100 μL of 0.18 N H2SO4. Within 10 minutes, the absorbance at 450/650 nm was read on a microplate reader (Spectra Max 340, Molecular Devices, Inc.). The positivity cut-off was determined by calculating the mean optical density (OD) ± 3 SD of 30 HEV-negative pig serum from Thailand.
Nipah virus serologic analysis.
Serum samples were initially screened for antibodies to NiV by using an indirect ELISA with irradiation-inactivated virus extracted as a soluble lysate from infected cells by treatment with a non-ionic detergent.41,42 Serum samples that showed positive or inconclusive test results were tested by using a microtiter virus neutralization test42,43 with a Malaysian isolate of NiV. Any neutralization at or beyond the initial one in two serum dilution was regarded as positive.
Swine influenza virus serologic analysis.
Serum samples were tested by using a competition ELISA44 previously used to detect antibody to influenza nucleoprotein (NP) in avian and equine serum samples. In brief, plates were coated with influenza NP derived from yeast cells transfected to express a recombinant long form of influenza A virus NP. Coated plates were first exposed to test serum diluted 1:10 and then to monoclonal antibody against NP (H16-l10-4R5). Washed plates were exposed to anti-mouse horseradish peroxidase conjugate, and binding of conjugate was assessed by 3,3′,5,5′-tetramethylbenzidine substrate conversion. Serum causing ≥ 40% inhibition of the monoclonal antibody were then tested for HI antibody to swine influenza subtypes A/California/07/2009 (pandemic H1N1), A/swine/Ratchaburi/2000 H1N1 (H1N1), and A/swine/Nakhon Pathom/2002 (H3N2). Serum samples were tested in accordance with World Organisation for Animal Health protocols.45 In brief, serum samples were treated with receptor-destroying enzyme and heated for 30 minutes at 56°C to remove non-specific inhibitors, adsorbed with packed chicken erythrocytes to remove non-specific agglutinins, and assessed in the HI by using 4 HA units. Positive serum samples were recognized as those with an HI titer ≥ 40.
Data analysis.
Seroprevalence was calculated as the proportion of serum samples with a positive test result in the sampled population. Pearson's chi-square test and Fisher's exact test were used to explore associations between infection status, as measured by JEV HI and HEV ELISA, and location of the slaughterhouse, age and breed of pigs, production system at last point of sale, and month of slaughter. Fisher's exact test was used to explore associations for JEV IgM positivity because of small numbers in the respective contingency tables.
Results
Characteristics of survey pig population.
Seven hundred twenty-nine and 724 serum samples were tested at AFRIMS for antibodies to JEV and HEV, respectively; two samples were mislabeled and excluded from the analysis. Seven hundred twenty-six serum samples were tested at the CSIRO Australian Animal Health Laboratory for SIV and NiV; seven samples were mislabeled and excluded from the analysis. Complete data were collected for location of slaughterhouse and the collection date, but only partial data were available for pig age, breed, and production system at last point of sale (Table 1 and Table 2). The median age was 12 months (25–75 percentile range = 8–16 months) for 656 pigs for which age data were available. Most (83.9%) slaughtered animals were indigenous breed swayback black pigs, and most (72.4%) were purchased by slaughter traders from a penned production system. The last point of sale provides no indication of the production systems encountered during the life of the animals.
Table 1.
Seroprevalence of antibodies against Japanese encephalitis virus (JEV) and hepatitis E virus (HEV) during May–September 2008 (wet season months) in Oudomxay and Xiengkhuang Provinces, Laos
| Population characteristic | JEV serology | HEV serology | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. tested | JEV HI assay | JEV IgM ELISA | No. tested | HEV IgG ELISA | |||||||
| % Positive | 95% CI | P | % Positive | 95% CI | P | % Positive | 95% CI | P | |||
| Province | |||||||||||
| Oudomxay | 143 | 90.2 | 85.3–95.1 | < 0.001 | 7.7 | 3.3–12.1 | 0.082* | 141 | 29.8 | 22.1–37.4 | 0.958 |
| Xiengkhuang | 186 | 70.4 | 63.8–77.0 | 3.2 | 0.7–5.8 | 183 | 30.1 | 23.3–36.8 | |||
| Collection date, 2008 | |||||||||||
| May | 40 | 77.5 | 64.0–91.0 | 0.148 | 2.5 | 0.0–7.6 | 0.694* | 39 | 35.9 | 20.1–51.7 | 0.008 |
| June | 144 | 76.4 | 69.4–83.4 | 6.3 | 2.2–10.3 | 141 | 39.0 | 30.9–47.2 | |||
| July | 84 | 78.6 | 69.6–87.5 | 7.1 | 1.5–12.8 | 83 | 18.1 | 9.6–26.5 | |||
| August | 38 | 78.9 | 65.4–92.5 | 2.6 | 0.0, 8.0 | 38 | 21.1 | 7.5–34.6 | |||
| September | 23 | 100 | – | 0.0 | – | 23 | 21.7 | 3.5–40.0 | |||
| Breed | |||||||||||
| Indigenous | 191 | 80.6 | 0.75–0.86 | 0.898 | 6.3 | 2.8–9.8 | 0.499* | 190 | 35.8 | 28.9–42.7 | 0.003 |
| Exotic | 68 | 82.4 | 73.1–91.6 | 2.9 | 0.0–7.1 | 66 | 13.6 | 5.1–22.1 | |||
| Cross-breed | 18 | 77.8 | 56.5–99.1 | 0.0 | – | 16 | 31.3 | 5.7–56.8 | |||
| Age (months) | |||||||||||
| 4–6 | 69 | 69.6 | 58.4–80.7 | 0.017 | 11.6 | 3.8–19.3 | 0.022* | 68 | 41.2 | 29.2–53.2 | 0.099 |
| 7–12 | 173 | 85.0 | 79.6–90.3 | 2.9 | 0.4–5.4 | 170 | 27.1 | 20.3–33.8 | |||
| > 12 | 23 | 87.0 | 72.1–100.0 | 4.3 | 0.0–13.4 | 23 | 34.8 | 13.7, 55.8 | |||
| Production type | |||||||||||
| Penned | 257 | 81.7 | 77.0–86.5 | 0.161* | 5.4 | 2.7–8.2 | 1.000* | 253 | 30.0 | 24.4–35.7 | 0.32* |
| Free range | 4 | 50.0 | 0.0–100.0 | 0.0 | – | 4 | 0.0 | – | |||
| Mixed | 0 | – | – | – | – | – | – | ||||
By Fisher's exact test (all other P values were calculated by using the chi-square test).
HI = hemagglutination inhibition; ELISA = enzyme-linked immunosorbent assay; CI = confidence interval.
Table 2.
Seroprevalence of antibodies against Japanese encephalitis virus (JEV) and hepatitis E virus (HEV) in Luangprabang and Huaphan Provinces during October 2008–January 2009 (dry season months), Laos
| Population characteristic | JEV serology* | HEV serology | ||||||
|---|---|---|---|---|---|---|---|---|
| No. tested | JEV HI assay | No. tested | HEV IgG ELISA | |||||
| % Positive | 95% CI | P | % Positive | 95% CI | P | |||
| Province | ||||||||
| Luangprabang | 209 | 59.8 | 53.1–66.5 | < 0.001 | 209 | 12.4 | 7.9–17.0 | 0.402 |
| Huaphan | 189 | 83.6 | 78.3–88.9 | 189 | 15.3 | 10.2–20.5 | ||
| Collection date | ||||||||
| October 2008 | 14 | 78.6 | 54.0–100.0 | 0.275 | 14 | 14.3 | 0.0–35.3 | 0.001 |
| November 2008 | 113 | 70.8 | 62.3–79.3 | 113 | 21.2 | 13.6–28.9 | ||
| December 2008 | 107 | 64.5 | 55.3–73.7 | 107 | 2.8 | 0.0–6.0 | ||
| January 2009 | 164 | 75.0 | 68.3–81.7 | 164 | 15.9 | 10.2–21.5 | ||
| Breed | ||||||||
| Indigenous | 367 | 70.6 | 65.9–75.2 | 0.987 | 367 | 14.4 | 10.8–18.1 | 0.370 |
| Exotic | 10 | 70.0 | 35.4–100.0 | 10 | 10.0 | 0.0–32.6 | ||
| Cross-breed | 11 | 72.7 | 41.3–100.0 | 11 | 0.0 | – | ||
| Age (months) | ||||||||
| 4–6 | 28 | 78.6 | 62.4–94.8 | 0.637 | 28 | 3.6 | 0.0–10.9 | 0.161 |
| 7–12 | 169 | 71.6 | 64.7–78.5 | 169 | 16.6 | 10.9–22.2 | ||
| > 12 | 193 | 69.9 | 63.4–76.5 | 193 | 13.0 | 8.2–17.7 | ||
| Production type | ||||||||
| Penned | 206 | 77.2 | 71.4–83.0 | 0.001 | 206 | 13.1 | 8.5–17.8 | 0.919 |
| Free range | 167 | 60.5 | 53.0–68.0 | 167 | 14.4 | 9.0–19.7 | ||
| Mixed | 6 | 100.0 | – | 6 | 16.7 | 0.0–59.9 | ||
All serum samples collected in Luangprabang and Huaphan Provinces were not reactive in the JEV IgM ELISA.
HI = hemagglutination inhibition; ELISA = enzyme-linked immunosorbent assay; CI = confidence interval.
Japanese encephalitis virus serologic analysis.
Antibodies to JEV were detected by HI in 543 (74.7%) of 727 (95% confidence interval [CI] = 71.5–77.9%), of which inhibition titers of 20, 40, and ≥ 80 were observed in 26 (3.6%), 55 (7.6%) and 462 (63.6%) pigs, respectively. In Oudomxay and Xiengkhuang Provinces, where samples were collected in the wet season months, there was a significantly lower seroprevalence of antibodies against JEV detected by HI in pigs in Xiengkhuang Province and in pigs 4–6 months of age. In contrast, pigs 4–6 months of age had the highest prevalence of IgM against JEV (Table 1). In Luangprabang and Huaphan Provinces, where samples were collected in the dry season months, there was a significantly lower seroprevalence of antibodies against JEV detected by HI in pigs in Luangprabang Province and in pigs raised in a free range production system (Table 2). IgM against JEV was not detected in samples collected from Luangprabang and Huaphan Provinces.
IgM was detected in 17 (5.2%) of 329 pigs (95% CI = 2.7–7.6%) in Oudomxay and Xiengkhuang Provinces, and comprised 11 pigs sampled from Oudomxay Province and 6 pigs sampled from Xiengkhouang Province. Fifteen of the 17 IgM-positive serum samples had HI titers ≥ 80, and the remaining two samples had HI titers of 40. Age data were available for 265 of 329 pigs, and peak seroprevalence (11.6%) was observed in the youngest age class of animals (Table 1) and was 20.7% (6 of 29) and 5.0% (2 of 40) of 4–6 month-old pigs in Oudomxay and Xiengkhuang Provinces, respectively.
Hepatitis E virus serologic analysis.
Seven hundred twenty-two serum samples were analyzed for antibodies against HEV by ELISA, and 152 (21.1%) had an OD ≥ 0.500 and were considered positive. One hundred sixty-three (22.6%) samples had an inconclusive borderline test result (OD > 0.260 and < 0.500); the remaining 407 (56.4%) samples were negative for antibodies to HEV (OD < 0.260). In Oudomxay and Xiengkhuang Provinces, where samples were collected in the wet season months, there was no observed spatial difference in seroprevalence. Seroprevalence was significantly higher in the early stages of the wet season (May and June) and significantly higher in indigenous and cross-breeds than in exotic breed pigs (Table 1). In Luangprabang and Huaphan Provinces, where samples were collected in the dry season, there was no observed spatial difference in seroprevalence and no observed difference for pig breeds. However, there was a significant decrease in seroprevalence for December 2008 (Table 2). In the wet season collection sites, seroprevalence peaked in 4–6-month-old pigs (41.2%) (Table 1). However, the reverse was observed for the dry season collection sites where 4–6-month-old pigs had the lowest seroprevalence (3.6%) (Table 2).
Nipah virus serologic analysis.
Seven hundred nineteen serum samples were tested by using the NiV comparative ELISA, of which 716 (99.6%) were negative and three (0.4%) had an inconclusive test result. The three ELISA inconclusive serum samples were tested by using the NiV neutralization assay, of which two samples were negative and one sample was toxic to the cell line.
Swine influenza virus serologic analysis.
Twenty-three (3.2%) of 719 pig serum samples were reactive in the ELISA, of which 13 (1.8%) had a percent inhibition > 60 and were considered samples with positive results. The remaining 10 samples had a PI of 40–60 and were considered samples with inconclusive results. Twenty ELISA-reactive serum samples were tested by HI for H3N2 (Nakorn Pathom); two samples were HI positive with titers 160 and 640 and the remaining 18 samples were negative by HI for H3N2. Twenty-one and 23 serum samples were tested by HI for H1N1 (Ratchaburi) and H1N1 (pandemic 2009 virus), respectively, and all were negative. Furthermore, 14 ELISA-negative serum samples were tested by HI for H3N2 (Nakorn Pathom) and two samples were HI positive. No ELISA-negative samples were tested by HI for H1N1 because of an insufficient volume of serum.
Discussion
This is the first study to report the seroprevalence of four viral zoonoses in the pig population of Laos. Previous published studies on JEV in Laos have focused on human populations,46,47 and our study represents the first assessment of the role pigs might play in transmission to humans in Laos. Previous swine HEV studies have been reported from Laos,15,48 and our survey supports and adds weight to the argument that pigs have a potential role in the natural history of human HEV disease. No published studies have reported seroprevalence of NiV and SIV in the pig population in Laos, and we provide strong evidence that pigs pose little risk for human NiV and SIV disease, at least at the time the survey was conducted. Antibodies against NiV were not detected, and only a limited number of animals had serologic evidence of a previous SIV infection with a non-pathogenic subtype.
This study demonstrates unequivocally that JEV was widespread in the pig population of northern Laos, and a seroprevalence of 74.7% was indicative of a hyper-epizootic state. Pigs are not vaccinated against JEV in northern Laos, and these results represent natural transmission. Furthermore, maternal antibodies wane after two months,49 and the youngest pigs in our survey population were four months of age, indicating that detected antibodies were raised against active JEV infections rather than via passive immunity.
Antibodies to JEV were detected in all provinces, and significant differences in prevalence were observed between provinces for both temporal sampling frames. Because HI can detect antibodies against JEV in pigs up to three years post-infection50 and the median age at slaughter was 12 months, it was unlikely that the observed seroprevalence in the two temporal sampling frames was influenced by the timing of sample collection. The differences we encountered might have been caused by factors such as pig density, rice paddy production, and Culex mosquito abundance. This hypothesis was further supported by the finding that pigs purchased for slaughter from free-range production systems had lower seroprevalences than penned pigs, and free-range production systems were encountered predominantly in upland rice-growing areas with limited paddies. However, the observed prevalence in all four provinces was high.
Prevalence of IgM against JEV peaked in June and July, corresponding to the start of the wet season, and water filling of rice paddies providing suitable breeding conditions for Culex mosquitoes. In pigs, IgM is detected within 2–3 days post-infection and can be detected in serum for up to 3 weeks,35 which indicated that IgM-positive pigs we detected were recently infected and that peak transmission and greatest risk for human infection corresponds with the first half of the wet season. This peak in pigs in Laos was consistent with peak transmission to humans in Thailand in June and July 1983.51 Because we did not present a single sampling frame over a complete year, caution should be exercised in interpreting seasonal transmission patterns. However, although we would not expect highly active transmission in the dry season months because of a lack of mosquito breeding sites, the impact of irrigated rice production on Culex mosquito abundance in the dry season of northern Laos remains to be determined.
The IgM ELISA results for pigs provide limited evidence that JEV is not maintained in the pig population throughout the year, which is consistent with an epizootic pattern of transmission. This finding could be caused by a combination of relatively low animal densities,52 a short duration of viremia, ranging from 1 to 3 days,53 and a decrease in mosquito vector abundance in the dry season winter months. The migration patterns of ardeid birds could therefore have a strong influence on JEV transmission patterns, and several ardeid bird species breed in Laos during the wet season months and other species overwinter during the dry season months.29,54 The role of these migratory birds in maintaining JEV in an epizootic state in Laos warrants further investigation.
In pigs, the most clinically significant manifestation of JEV infection is reproductive failure in sows because of abortion and abnormal farrowing.6 The high seroprevalence of JEV in young pigs ≤ 6 months of age indicates that JEV would have little or no impact on the reproductive potential of local indigenous breed sows. Indigenous breed sows in Southeast Asia sexually mature at 6–8 months of age,55 and most sows in Laos would have protective immunity by the age of first estrus. However, the impact on the reproductive potential of indigenous breed boars may be more significant. Indigenous breed boars in Southeast Asia can reach sexual maturity at 2–3 months of age,55 and infection of sexually mature boars can cause infertility.6 Because the smallholder pig sector in Laos has low productivity,56,57 we believe that the effect of JEV on this pig producing sector warrants greater scrutiny, with particular reference to boar infertility.
Two recent swine HEV studies in Laos15,48 and the present study demonstrate the relative importance of pigs as a reservoir of human HEV disease. Blacksell and others15 observed a high seroprevalence of HEV in pigs sampled at provincial slaughterhouses in northern Laos; 85.7%, 47.1%, 60.0%, and 72.1% for Huaphan, Luangprabang, Oudomxay, and Xiengkhuang Provinces, respectively. These data are substantially higher than those observed during this current study; 29–30% was observed in Xiengkhuang and Oudomxay Provinces in the wet season and 12–15% in Huaphan and Luangprabang in the dry season. The difference may have been caused by seasonal variation because Blacksell and others15 observed high seroprevalence in Huaphan and Luangprabang Provinces when sampling was conducted in the wet season months. Further work will be required to confirm seasonal peaks of transmission. However, data for Oudomxay and Xiengkhuang Provinces provides evidence that the peak seroprevalence was at the start of the wet season.
Age-related seroprevalence in the current study peaked in 4–6-month-old pigs sampled in the wet season and in 7–12-month-old pigs sampled in the dry season. The combined temporal and age prevalence data indicates that young animals are an important reservoir of HEV at the beginning of the wet season. We can speculate that management of young animals differs from that of older animals and predisposes them to infection during the wet season. However, further research will be required to understand the specific production practices associated with increased risk of HEV transmission. The importance of young animals in the epidemiology of HEV in Laos was further demonstrated by Conlan and others,48 who observed that 11.6% of pigs ≤ 6 months of age were shedding virus during the dry season months (January–March). Only genotype 4 HEV has been recognized in northern Laos,48 and this same genotype has been identified as the most common cause of human HEV disease in southern and eastern China.16,17 The seroprevalence of human HEV in Laos has been estimated to be 16–18%, and 2–4% of acute hepatitis hospital admissions were caused by HEV.13,14 To date, no data exist that describe the genotypes causing human HEV disease in Laos, and work should now be undertaken to establish the source of human infections.
Nipah virus has been detected in Pteropid fruit bats in Malaysia, Cambodia, and Thailand by serologic analysis, virus isolation, or RNA amplification,24,26,58 and NiV antibodies have also been detected in fruit bats in Indonesia,25 providing evidence that NiV is endemic to Southeast Asia. The most human cases and deaths of any outbreak to date occurred during the outbreaks in Malaysia and Singapore in 1999, and pigs were the source of human infections, demonstrating the importance of pigs as an intermediary amplification host. We found no serologic evidence of NiV infection of pigs in Laos, but this finding does not confirm the absence of NiV in this country. Pteropid fruit bats are present in Laos and to better understand the epidemiology and risks of NiV, surveys of these competent reservoir host species will be required.
Serologic data for SIV indicated low levels of virus circulation in the pig survey population during May 2008–January 2009. We found no evidence of H1NI (Ratchaburi or pandemic 2009 strain) infection; only the H3N2 subtype was detected. We could only confirm HI positivity for 2 of 23 ELISA-reactive serum samples, both of which were inconclusive in the ELISA, indicating that the ELISA has not been fully optimized for pig serum or that different subtypes are circulating in pigs in Laos. The influenza ELISA is well defined for testing avian and equine serum but less well defined for testing pig serum. This finding was further evident in our finding that two ELISA-negative samples were positive for H3N2 by HI. Influenza HI is affected by the degree of homology between the assay virus and the virus to which animals have been exposed. It was possible that many of the 14 serum samples that were ELISA positive, but not HI positive, could represent animals with exposure to a sufficiently different but homologous subtype, or to an untested subtype such as H9 or H5. In southern China, near the border region with northern Laos, the H9 subtype has been shown to have a prevalence of 10.5%30 and could explain some of the discrepancies we observed between ELISA and HI results. However, the 2 of 14 ELISA-negative HI (H3N2)-positive serum samples suggests that additional work is required to define ELISA sensitivity with pig serum in Southeast Asia. The low frequency of antibody to SIV may also result from a low-density pig population acting as a natural barrier to maintenance of virus endemicity and therefore to timing of the test cohort relative to the opportunity for periodic spread of the virus.
We provide evidence that pigs are an important reservoir of the viral zoonoses JEV and HEV in Laos, and limited evidence suggests the early months of the monsoonal wet season (May–July) coincide with peak transmission in pigs and greatest risk for human disease. Our study supports the need for continued surveillance of pig-associated viral zoonoses and the integration of human and veterinary public health authorities to control these important diseases.
ACKNOWLEDGMENTS
We thank district, provincial, and national government staff from the Lao Department of Livestock and Fisheries, Ministry of Agriculture and Forestry, particularly Lapinh Phithacthep, Vilaywan Soukvilay, Manivanh Phouarawan, and Vilayphet Viravong (National Animal Health Centre) for their critical contributions to the study.
Disclaimer: The opinions or assertions contained herein are the private views of the authors, and not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
Footnotes
Financial support: This study was supported by the Australian Centre for International Agricultural Research (project no. AH2006/161), and the laboratory work at the Armed Forces Research Institute of Medical Sciences was partially supported by U.S. Department of State Bioengagement Program. James V. Conlan was supported by a Murdoch University Research Studentship award.
Author addresses: James V. Conlan, Stanley Fenwick, and R. C. Andrew Thompson, School of Veterinary and Biomedical Sciences, Murdoch University, South Street, Murdoch, Western Australia 6150, Australia, E-mails: j.conlan@murdoch.edu.au, stanley_fenwick@dai.com, and a.thompson@murdoch.edu.au. Khamphouth Vongxay, Department of Livestock and Fisheries, Ministry of Agriculture and Forestry, Vientiane, Laos, E-mail: kamputvongxay@yahoo.com. Richard G. Jarman and Robert V. Gibbons, Department of Virology Armed Forces Research Institute of Medical Sciences United States Army Medical Component, Bangkok, Thailand, E-mails: richard.jarman@afrims.org and robert.gibbons@afrims.org. Ross A. Lunt, Commonwealth Scientific and Industrial Research Organisation Livestock Industries, Australian Animal Health Laboratory, Geelong, Victoria, Australia, E-mail: ross.lunt@csiro.au. Stuart D. Blacksell, Mahidol-Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Ratchathewi District, Bangkok, Thailand, E-mail: stuart@tropmedres.ac.
References
- 1.Erlanger TE, Weiss S, Keiser J, Utzinger J, Wiedenmayer K. Past, present, and future of Japanese encephalitis. Emerg Infect Dis. 2009;15:1–7. doi: 10.3201/eid1501.080311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, Ksiazek TG, Rollin PE, Zaki SR, Shieh W, Goldsmith CS, Gubler DJ, Roehrig JT, Eaton B, Gould AR, Olson J, Field H, Daniels P, Ling AE, Peters CJ, Anderson LJ, Mahy BW. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- 3.Kitikoon P, Sreta D, Tuanudom R, Amonsin A, Suradhat S, Oraveerakul K, Poovorawan Y, Thanawongnuwech R. Serological evidence of pig-to-human influenza virus transmission on Thai swine farms. Vet Microbiol. 2011;148:413–418. doi: 10.1016/j.vetmic.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Wibawa ID, Suryadarma IG, Mulyanto Tsuda F, Matsumoto Y, Ninomiya M, Takahashi M, Okamoto H. Identification of genotype 4 hepatitis. E virus strains from a patient with acute hepatitis E and farm pigs in Bali, Indonesia. J Med Virol. 2007;79:1138–1146. doi: 10.1002/jmv.20904. [DOI] [PubMed] [Google Scholar]
- 5.van den Hurk AF, Ritchie SA, Mackenzie JS. Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- 6.Joo HS, Platt KB. In: Diseases of Swine. Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, editors. Ames, IA: Blackwell Publishing; 2006. pp. 875–889. (Japanese encephalitis and West Nile viruses). [Google Scholar]
- 7.Messerli P, Heinimann A, Epprecht M, Phonesaly S, Thiraka C, Minot N, editors. Socio-Economic Atlas of the Lao PDR: An Analysis Based on the 2005 Population and Housing Census. Bern, Switzerland: Swiss National Center of Competence in Research North-South, University of Bern and Vientiane, Laos Geographica Bernensia; 2008. [Google Scholar]
- 8.Blacksell SD. Classical Swine Fever and Emerging Viral Diseases in Southeast Asia; Proceedings of an International Workshop, Vientiane, Lao PDR; September 19–22, 1999; Canberra, Australia. 2000. ACIAR Proceedings No. 94. [Google Scholar]
- 9.Millar J, Photakoun V. Livestock development and poverty alleviation: revolution or evolution for upland livelihoods in Lao PDR? Int J Agr Sustain. 2008;6:89–102. [Google Scholar]
- 10.Meng XJ. Recent advances in hepatitis. E virus. J Viral Hepat. 2010;17:153–161. doi: 10.1111/j.1365-2893.2009.01257.x. [DOI] [PubMed] [Google Scholar]
- 11.Meng XJ. Hepatitis E virus: animal reservoirs and zoonotic risk. Vet Microbiol. 2009;140:256–265. doi: 10.1016/j.vetmic.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu L, Li C, Hagedorn CH. Phylogenetic analysis of global hepatitis. E virus sequences: genetic diversity, subtypes and zoonosis. Rev Med Virol. 2006;16:5–36. doi: 10.1002/rmv.482. [DOI] [PubMed] [Google Scholar]
- 13.Corwin AL, Tien NT, Bounlu K, Winarno J, Putri MP, Laras K, Larasati RP, Sukri N, Endy T, Sulaiman HA, Hyams KC. The unique riverine ecology of hepatitis. E virus transmission in South-East Asia. Trans R Soc Trop Med Hyg. 1999;93:255–260. doi: 10.1016/s0035-9203(99)90014-7. [DOI] [PubMed] [Google Scholar]
- 14.Syhavong B, Rasachack B, Smythe L, Rolain JM, Roque-Afonso AM, Jenjaroen K, Soukkhaserm V, Phongmany S, Phetsouvanh R, Soukkhaserm S, Thammavong T, Mayxay M, Blacksell SD, Barnes E, Parola P, Dussaix E, Raoult D, Humphreys I, Klenerman P, White NJ, Newton PN. The infective causes of hepatitis and jaundice amongst hospitalised patients in Vientiane, Laos. Trans R Soc Trop Med Hyg. 2010;104:475–483. doi: 10.1016/j.trstmh.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blacksell SD, Myint KS, Khounsy S, Phruaravanh M, Mammen MP, Jr, Day NP, Newton PN. Prevalence of hepatitis E virus antibodies in pigs: implications for human infections in village-based subsistence pig farming in the Lao PDR. Trans R Soc Trop Med Hyg. 2007;101:305–307. doi: 10.1016/j.trstmh.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, He Y, Wang H, Shen Q, Cui L, Wang X, Shao S, Hua X. Hepatitis E virus genotype diversity in eastern China. Emerg Infect Dis. 2010;16:1630–1632. doi: 10.3201/eid1610.100873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li RC, Ge SX, Li YP, Zheng YJ, Nong Y, Guo QS, Zhang J, Ng MH, Xia NS. Seroprevalence of hepatitis E virus infection, rural southern People's Republic of China. Emerg Infect Dis. 2006;12:1682–1688. doi: 10.3201/eid1211.060332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chua KB, Goh KJ, Wong KT, Kamarulzaman A, Tan PS, Ksiazek TG, Zaki SR, Paul G, Lam SK, Tan CT. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet. 1999;354:1257–1259. doi: 10.1016/S0140-6736(99)04299-3. [DOI] [PubMed] [Google Scholar]
- 19.Paton NI, Leo YS, Zaki SR, Auchus AP, Lee KE, Ling AE, Chew SK, Ang B, Rollin PE, Umapathi T, Sng I, Lee CC, Lim E, Ksiazek TG. Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet. 1999;354:1253–1256. doi: 10.1016/S0140-6736(99)04379-2. [DOI] [PubMed] [Google Scholar]
- 20.Harit AK, Ichhpujani RL, Gupta S, Gill KS, Lal S, Ganguly NK, Agarwal SP. Nipah/Hendra virus outbreak in Siliguri, West Bengal, India in 2001. Indian J Med Res. 2006;123:553–560. [PubMed] [Google Scholar]
- 21.Harcourt BH, Lowe L, Tamin A, Liu X, Bankamp B, Bowden N, Rollin PE, Comer JA, Ksiazek TG, Hossain MJ, Gurley ES, Breiman RF, Bellini WJ, Rota PA. Genetic characterization of Nipah virus, Bangladesh, 2004. Emerg Infect Dis. 2005;11:1594–1597. doi: 10.3201/eid1110.050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackenzie JS. Emerging zoonotic encephalitis viruses: lessons from Southeast Asia and Oceania. J Neurovirol. 2005;11:434–440. doi: 10.1080/13550280591002487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson JG, Rupprecht C, Rollin PE, An US, Niezgoda M, Clemins T, Walston J, Ksiazek TG. Antibodies to Nipah-like virus in bats (Pteropus lylei), Cambodia. Emerg Infect Dis. 2002;8:987–988. doi: 10.3201/eid0809.010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynes JM, Counor D, Ong S, Faure C, Seng V, Molia S, Walston J, Georges-Courbot MC, Deubel V, Sarthou JL. Nipah virus in Lyle's flying foxes, Cambodia. Emerg Infect Dis. 2005;11:1042–1047. doi: 10.3201/eid1107.041350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sendow I, Field HE, Adjid A, Ratnawati A, Breed AC, Darminto Morrissy C, Daniels P. Screening for Nipah virus infection in West Kalimantan Province, Indonesia. Zoonoses Public Health. 2009;57:499–503. doi: 10.1111/j.1863-2378.2009.01252.x. [DOI] [PubMed] [Google Scholar]
- 26.Wacharapluesadee S, Lumlertdacha B, Boongird K, Wanghongsa S, Chanhome L, Rollin P, Stockton P, Rupprecht CE, Ksiazek TG, Hemachudha T. Bat Nipah virus, Thailand. Emerg Infect Dis. 2005;11:1949–1951. doi: 10.3201/eid1112.050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luby SP, Rahman M, Hossain MJ, Blum LS, Husain MM, Gurley E, Khan R, Ahmed BN, Rahman S, Nahar N, Kenah E, Comer JA, Ksiazek TG. Foodborne transmission of Nipah virus, Bangladesh. Emerg Infect Dis. 2006;12:1888–1894. doi: 10.3201/eid1212.060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurley ES, Montgomery JM, Hossain MJ, Bell M, Azad AK, Islam MR, Molla MA, Carroll DS, Ksiazek TG, Rota PA, Lowe L, Comer JA, Rollin P, Czub M, Grolla A, Feldmann H, Luby SP, Woodward JL, Breiman RF. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerg Infect Dis. 2007;13:1031–1037. doi: 10.3201/eid1307.061128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duckworth JW, Salter RE, Khounboline K. Wildlife of Lao PDR: 1999 Status Report. Vientiane, Laos: IUCN-The World Conservation Union/Wildlife Conservation Society/Centre for Protected Areas and Watershed Management; 1999. [Google Scholar]
- 30.Liu W, Wei MT, Tong Y, Tang F, Zhang L, Fang L, Yang H, Cao WC. Seroprevalence and genetic characteristics of five subtypes of influenza A viruses in the Chinese pig population: a pooled data analysis. Vet J. 2011;187:200–206. doi: 10.1016/j.tvjl.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Chutinimitkul S, Thippamom N, Damrongwatanapokin S, Payungporn S, Thanawongnuwech R, Amonsin A, Boonsuk P, Sreta D, Bunpong N, Tantilertcharoen R, Chamnanpood P, Parchariyanon S, Theamboonlers A, Poovorawan Y. Genetic characterization of H1N1, H1N2 and H3N2 swine influenza virus in Thailand. Arch Virol. 2008;153:1049–1056. doi: 10.1007/s00705-008-0097-7. [DOI] [PubMed] [Google Scholar]
- 32.Shinde V, Bridges CB, Uyeki TM, Shu B, Balish A, Xu X, Lindstrom S, Gubareva LV, Deyde V, Garten RJ, Harris M, Gerber S, Vagasky S, Smith F, Pascoe N, Martin K, Dufficy D, Ritger K, Conover C, Quinlisk P, Klimov A, Bresee JS, Finelli L. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N Engl J Med. 2009;360:2616–2625. doi: 10.1056/NEJMoa0903812. [DOI] [PubMed] [Google Scholar]
- 33.Boltz DA, Douangngeun B, Sinthasack S, Phommachanh P, Rolston S, Chen H, Guan Y, Malik Peiris JS, Smith GJD, Webster RG. H5N1 Influenza virus in Lao People's Democratic Republic. Emerg Infect Dis. 2006;12:1593–1595. doi: 10.3201/eid1210.060658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarke DH, Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 35.Burke DS, Tingpalapong M, Elwell MR, Paul PS, Van Deusen RA. Japanese encephalitis virus immunoglobulin M antibodies in porcine sera. Am J Vet Res. 1985;46:2054–2057. [PubMed] [Google Scholar]
- 36.Burke DS, Nisalak A. Detection of Japanese encephalitis virus immunoglobulin M antibodies in serum by antibody capture radioimmunoassay. J Clin Microbiol. 1982;15:353–361. doi: 10.1128/jcm.15.3.353-361.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burke DS, Nisalak A, Ussery MA. Antibody capture immunoassay detection of Japanese encephalitis virus immunoglobulin M and G antibodies in cerebrospinal fluid. J Clin Microbiol. 1982;16:1034–1042. doi: 10.1128/jcm.16.6.1034-1042.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burke DS, Nisalak A, Ussery MA, Laorakpongse T, Chantavibul S. Kinetics of IgM and IgG responses to Japanese encephalitis virus in human serum and cerebrospinal fluid. J Infect Dis. 1985;151:1093–1099. doi: 10.1093/infdis/151.6.1093. [DOI] [PubMed] [Google Scholar]
- 39.Innis BL, Seriwatana J, Robinson RA, Shrestha MP, Yarbough PO, Longer CF, Scott RM, Vaughn DW, Myint KS. Quantitation of immunoglobulin to hepatitis E virus by enzyme immunoassay. Clin Diagn Lab Immunol. 2002;9:639–648. doi: 10.1128/CDLI.9.3.639-648.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Zhang H, Li Z, Gu W, Lan H, Hao W, Ling R, Li H, Harrison TJ. Detection of sporadic cases of hepatitis E virus (HEV) infection in China using immunoassays based on recombinant open reading frame 2 and 3 polypeptides from HEV genotype 4. J Clin Microbiol. 2001;39:4370–4379. doi: 10.1128/JCM.39.12.4370-4379.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daniels P, Ksiazek T, Eaton BT. Laboratory diagnosis of Nipah and Hendra virus infections. Microbes Infect. 2001;3:289–295. doi: 10.1016/s1286-4579(01)01382-x. [DOI] [PubMed] [Google Scholar]
- 42.OIE Chapter 2.9.6. Hendra and Nipah Virus Diseases. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2010. http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.09.06_HENDRA_&_NIPAH_FINAL.pdf Office International des Epizooties.
- 43.Middleton DJ, Westbury HA, Morrissy CJ, van der Heide BM, Russell GM, Braun MA, Hyatt AD. Experimental Nipah virus infection in pigs and cats. J Comp Pathol. 2002;126:124–136. doi: 10.1053/jcpa.2001.0532. [DOI] [PubMed] [Google Scholar]
- 44.Sergeant ES, Kirkland PD, Cowled BD. Field evaluation of an equine influenza ELISA used in New South Wales during the 2007 Australian outbreak response. Prev Vet Med. 2009;92:382–385. doi: 10.1016/j.prevetmed.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 45.OIE Chapter 2.8.8. Swine Influenza. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2010. http://www.oie.int/eng/normes/mmanual/2008/pdf/2.08.08_SWINE_INFLUENZA.pdf Office International des Epizooties.
- 46.Vallee J, Dubot-Peres A, Ounaphom P, Sayavong C, Bryant JE, Gonzalez JP. Spatial distribution and risk factors of dengue and Japanese encephalitis virus infection in urban settings: the case of Vientiane, Lao PDR. Trop Med Int Health. 2009;14:1134–1142. doi: 10.1111/j.1365-3156.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 47.Vongxay P. Epidemiology of Japanese encephalitis virus in Lao PDR. Southeast Asian J Trop Med Public Health. 1995;26:28–30. [Google Scholar]
- 48.Conlan JV, Jarman RG, Vongxay K, Chinnawirotpisan P, Melendrez MC, Fenwick S, Thompson RC, Blacksell SD. Hepatitis E virus is prevalent in the pig population of Lao People's Democratic Republic and evidence exists for homogeneity with Chinese genotype 4 human isolates. Infect Genet Evol. 2011;11:1306–1311. doi: 10.1016/j.meegid.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 49.Scherer WF, Moyer JT, Izumi T. Immunologic studies of Japanese encephalitis virus in Japan. V. Maternal antibodies, antibody responses and viremia following infection of swine. J Immunol. 1959;83:620–626. [PubMed] [Google Scholar]
- 50.Geevarghese G, Shaikh BH, Jacob PG, Bhat HR. Persistence of haemagglutination-inhibition antibodies to JE and WN viruses in naturally infected domestic pigs in Karnataka State, India. Acta Virol. 1994;38:235–237. [PubMed] [Google Scholar]
- 51.Burke DS, Chatiyanonda K, Anandrik S, Nakornsri S, Nisalak A, Hoke CH., Jr Improved surveillance of Japanese encephalitis by detection of virus-specific IgM in desiccated blood specimens. Bull World Health Organ. 1985;63:1037–1042. [PMC free article] [PubMed] [Google Scholar]
- 52.FAO . Livestock Sector Brief: Lao PDR. Rome, Italy: Food and Agriculture Organisation of the United Nations; 2005. [Google Scholar]
- 53.Williams DT, Daniels PW, Lunt RA, Wang LF, Newberry KM, Mackenzie JS. Experimental infections of pigs with Japanese encephalitis virus and closely related Australian flaviviruses. Am J Trop Med Hyg. 2001;65:379–387. doi: 10.4269/ajtmh.2001.65.379. [DOI] [PubMed] [Google Scholar]
- 54.Kushlan JA, Hancock J. The Herons. Oxford, United Kingdom: Oxford University Press; 2005. [Google Scholar]
- 55.Dang-Nguyen TQ, Tich NK, Nguyen BX, Ozawa M, Kikuchi K, Manabe N, Ratky J, Kanai Y, Nagai T. Introduction of various Vietnamese indigenous pig breeds and their conservation by using assisted reproductive techniques. J Reprod Dev. 2010;56:31–35. doi: 10.1262/jrd.09-165k. [DOI] [PubMed] [Google Scholar]
- 56.Conlan J, Khounsy S, Phithakhep L, Phruaravanh M, Soukvilai V, Colling A, Wilks C, Gleeson L. Pig production and health in Bolikhamxay Province, Lao PDR. In: Conlan JV, Blacksell SD, Morrissy CJ, Colling A, editors. Management of Classical Swine Fever and Foot-and-Mouth Disease in Lao PDR; Proceedings of an International Workshop Held in Vientiane, Lao PDR; November 20–21, 2006; 2008. ACIAR Proceedings No. 128, 98. [Google Scholar]
- 57.Phengsavanh P, Ogle B, Stur W, Frankow-Lindberg BE, Lindberg JE. Feeding and performance of pigs in smallholder production systems in northern Lao PDR. Trop Anim Health Prod. 2010;42:1627–1633. doi: 10.1007/s11250-010-9612-4. [DOI] [PubMed] [Google Scholar]
- 58.Yob JM, Field H, Rashdi AM, Morrissy C, van der Heide B, Rota P, bin Adzhar A, White J, Daniels P, Jamaluddin A, Ksiazek T. Nipah virus infection in bats (order Chiroptera) in peninsular Malaysia. Emerg Infect Dis. 2001;7:439–441. doi: 10.3201/eid0703.010312. [DOI] [PMC free article] [PubMed] [Google Scholar]