Abstract
Because malaria is still a significant problem worldwide, additional control methods need to be developed. The Plasmodium sporozoite is a good target for control measures because it displays dual infectivity for both mosquito and vertebrate host tissues. The Plasmodium falciparum gene, PFE0565w, was chosen as a candidate for study based on data from PlasmoDB, the Plasmodium database, indicating that it is expressed both at the transcriptional and protein levels in sporozoites, likely encodes a putative surface protein, and may have a potential role in the invasion of host tissues. Additional sequence analysis shows that the PFE0565w protein has orthologs in other Plasmodium species, but none outside of the genus Plasmodium. PFE0565w expresses transcript during both the sporozoite and erythrocytic stages of the parasite life cycle, where an alternative transcript was discovered during the erythrocytic stages. Data show that transcript is not present during axenic exoerythrocytic stages. Despite transcript being present in several life cycle stages, the PFE0565w protein is present only during the salivary gland sporozoite stage. Because the PFE0565w protein is present in salivary gland sporozoites, it could be a novel candidate for a pre-erythrocytic stage vaccine.
Introduction
Malaria is a devastating disease caused by Plasmodium parasites that is still a significant problem around the world due, in part, to the development of insecticide-resistant mosquitoes and drug-resistant Plasmodium parasites.1 Because of malaria's significant impact on human health and welfare, it is critical for improved and/or new control methods to be developed, such as new drugs and the creation of vaccines. Plasmodium sporozoites are a promising target for the development of malaria control methods because they have dual infectivity for tissues of both the mosquito vector and vertebrate host. In addition, many promising vaccines using Plasmodium proteins were generated using/targeting surface and/or secreted proteins because many of these proteins have been shown to be important in development and/or invasion of host tissues.2 For example, the circumsporozoite protein is a well-characterized sporozoite surface protein that is essential for proper parasite development, invasion of both mosquito salivary glands and vertebrate host tissues, and is one of the components of one of the most promising malaria vaccines to date, the RTS, S vaccine.3–8 Thus, to continue the search for Plasmodium proteins that could be used in efforts to control malaria, an in silico data mining technique was used to choose the Plasmodium falciparum gene, PFE0565w, as a candidate for study. PFE0565w was selected based on data collected from PlasmoDB, the Plasmodium database, indicating that this gene likely encodes a putative surface protein, which is expressed in the sporozoite both at the transcriptional and protein levels.9,10
The experiments described herein further characterize the gene, PFE0565w, and the protein it produces by determining its transcript profile, protein expression profile, and localization. The PFE0565w transcripts are present during both sporozoite stages and erythrocytic stages of the life cycle, but are not present during axenic exoerythrocytic stages, as demonstrated by reverse transcriptase-polymerase chain reaction (RT-PCR). During the erythrocytic stages, including gametocytes, PFE0565w produces an alternative transcript. Despite transcript presence during many life cycle stages, the PFE0565w protein is only detected in salivary gland sporozoites, as indicated by Western blot analysis, immunofluorescent assays, and green fluorescent protein (GFP)-trafficking studies. These studies have generated data about the transcript and protein expression patterns of another novel P. falciparum gene/protein and may also provide the field with a new pre-erythrocytic stage vaccine candidate.
Materials and Methods
Parasite maintenance, parasite transmission, and cell cultures.
Plasmodium falciparum strain NF54 was used for the following experiments. Plasmodium falciparum cultures were maintained in human blood (O+ male, BioChemed Services, Winchester, VA) at a 6% hematocrit in complete culture medium, RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 25 mM HEPES (Invitrogen, Grand Island, NY), 0.5% Albumax (Invitrogen), and 0.005% hypoxanthine (Sigma, St. Louis, MO). The medium was changed every 48 hours and the parasites were kept in a gas mixture (3% O2, 3% CO2, and 94% N2) at 37°C.11 Cultures were diluted/split to prevent the parasitemia from becoming too high by addition of fresh, washed 50% blood (blood washed three times with sterile RPMI and diluted 50:50 with RPMI), maintaining a 6% hematocrit level. The use of human blood was in compliance with federal guidelines and institutional policies. All experiments described throughout this work were approved by the University of Missouri's Institutional Biosafety Committee (IBC), Institutional Review Board (IRB), and Institutional Animal Care and Use Committee (IACUC).
To obtain infected mosquitoes to study the parasite stages within the vector host, 4–5 day old, female Anopheles stephensi (details in next section) were exposed to P. falciparum-infected blood (1:1 ratio of infected blood and human serum), using induced gametocyte cultures. Gametocyte cultures were produced by setting a standard culture (described previously) at a parasitemia of between 0.5–1.0% and maintaining them in complete culture media supplemented with 10% human serum (A+ male, Interstate Blood Bank, Memphis, TN); however, instead of splitting the parasites with fresh red blood cells (RBCs), the culture was left undiluted such that a high parasitemia developed and the parasites became stressed. This was done for 16 days and resulted in a mixture of male and female gametocyte stages (I–V), with a majority of them being mature stages. The mosquitoes were fed the infected blood for ∼30 minutes, using a 37°C water-jacketed membrane feeding system. After the blood feed, only colony cages where at least 75% of the females fed were used and the infected mosquitoes were maintained in an incubator (Precision Low Temperature Illuminated Incubator 818, Thermo Fisher Scientific, Waltham, MA) at 26–27°C with 82–88% humidity using a light/dark cycle of 16 and 8 hours, respectively.
To obtain exoerythrocytic stages, axenic cultures (cultures without hepatocytes) were used. In brief, ∼5 × 104 salivary gland sporozoites were added to a well of a 48-well plate (Corning, Corning, NY) and allowed to incubate in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) and 500 U/mL and 500 μg/mL penicillin and streptomycin, respectively. These cultures were kept in a 37°C incubator with 5% CO2 for 24 hours before collection for transcript expression studies.12
Mosquito maintenance.
Anopheles stephensi mosquitoes were used for all studies and were reared using protocols available from the Malaria Research and Reference Reagent Resource Center (MR4). In brief, larvae and adults were maintained in an insectary with 78–85% humidity at ∼26–27°C on a light/dark cycle of 16 and 8 hours, respectively. Larvae were fed both a mixture of Fleischmann's 0.33 g yeast (ConAgra Foods, Omaha, NE) and 0.66 g micron (Sera Micron, Germany) per 50 mL water and game fish chow (Purina, St. Louis, MO). Adults were fed sucrose (0.3 M) ad libitum.
Selection of candidate gene, PFE0565w.
An in silico data mining procedure was used to select PFE0565w as a gene of interest. Briefly, PlasmoDB was used to search for P. falciparum proteins predicted to be expressed only by the sporozoite and contain a signal peptide, increasing its probability of being a surface and/or secreted protein.9,13 This process narrowed gene numbers down to 34 candidate genes. Next, additional sequence analysis programs available on the ExPASy Bioinformatics Resource Portal (www.expasy.org) and SoftBerry (www.softberry.com), such as PSORT and ProtComp, were used to verify that the proteins encoded by the genes were predicted to either be located on the surface and/or secreted by the parasite. Those proteins that were verified by these two programs to meet the required criteria (and were not proteins that had been studied or were currently being studied) became our genes of interest, including PFE0565w.
PFE0565w sequence analysis.
Using PlasmoDB, the full genomic DNA (gDNA), complimentary DNA (cDNA), and protein sequence of PFE0565w were obtained. A list of PFE0565w's orthologs was compiled using both PlasmoDB and the National Center for Biotechnology Information's (NCBI) BLAST analysis program.14 The ortholog sequences were then aligned using Vector NTI (Explorer or ContigExpress, Invitrogen). Additional sequence information for PFE0565w was obtained by using software programs such as TargetP, SignalP, PSORT II, WoLF PSORT, and PROSITE, all found using the ExPASy Bioinformatics Resource Portal (www.expasy.org).15
Isolation of P. falciparum-infected tissues for transcript and protein expression studies.
Oocyst sporozoites.
Anopheles stephensi were infected with P. falciparum as previously described. Ten days post-infection (PI), when oocyst sporozoites were mature using our laboratory conditions, 50 midguts were dissected from the abdomens of An. stephensi that had fed on a P. falciparum-infected blood meal. The tissues were placed into 50 μL 1X phosphate buffered saline (10X PBS, 0.2 M phosphate buffer, and 1.5 M NaCl pH 7.0, diluted 1:10 with Millipore water) (Millipore, Billerica, MA) in microcentrifuge tubes, snap-frozen in liquid nitrogen, and stored at −80°C until needed for RNA isolation.
Salivary gland sporozoites.
Fifty sets of An. stephensi salivary glands were dissected from mosquitoes that had fed on a P. falciparum-infected blood meal 14 days PI because sporozoites reside in the glands at this time under our laboratory conditions. The tissues were placed into 50 μL 1X PBS in microcentrifuge tubes, snap-frozen in liquid nitrogen, and stored at −80°C until needed for RNA isolation.
Exoerythrocytic stages.
For axenic exoerythrocytic stages, Trizol (Invitrogen) was directly added to the cultures to begin the process of RNA isolation after 24 hours of incubation.
Mixed erythrocytic stages and gametocytes.
Plasmodium falciparum cultures were maintained as previously described. Either mixed erythrocytic stage (ES) cultures (containing a mixture of rings, trophozoites, and schizonts) or 16-day old mixed gametocyte cultures (containing a mixture of stage I–V gametocytes, but with more mature forms present) were collected by centrifugation at 2,650 × g for 5 minutes. The infected RBCs were lysed with 0.05% saponin (Invitrogen) in complete culture medium for 3 minutes at room temperature (RT) and parasites collected by centrifugation for 5 minutes at 2,650 × g. Purified parasites were then washed once with RPMI 1640 medium and collected again by centrifugation as previously described. The parasite pellets were stored at −80°C until needed for either RNA isolation or Western blot analysis.
RNA/DNA isolation and transcriptional analysis by RT-PCR.
Total RNA was isolated from P. falciparum-infected tissues using a Trizol reagent-based protocol, following the manufacturer's instructions (Invitrogen). The samples were all DNase-treated (Promega), according to the manufacturer's instructions, to remove any contaminating gDNA. Approximately 2–3 μg of the DNase-treated RNA was used to synthesize cDNA using OligoDT primers from a SuperScript III First-Strand Synthesis System (Invitrogen), following the manufacturer's instructions.
Genomic DNA was isolated following the manufacturer's instructions using a DNeasy Blood and Tissue Kit (Qiagen, Carlsbad, CA) and used as a positive control for all RT-PCR experiments. The PFE0565w full-length gene-specific primers (5′-atgaagatgattaatattgg-3′ forward and 5′-tcacataaattcctgttgaattttg-3′ reverse) were used to amplify a 1,146 base pair (bp) DNA fragment in a PCR using 2.0 μL gDNA (∼100 ng total)/cDNA (∼1/10 total volume synthesized from above), 1.25 units GoTaq DNA Polymerase (Promega), 1X GoTaq Flexi Buffer, 1 mM MgCl2, 0.2 mM di-nucleotide tri-phosphate mix, and 0.5 μM primers. The PCR was conducted using conditions previously described, but with an annealing temperature of 53°C.16,17 For RT-PCR positive controls used during the axenic exoerythrocytic stages, primers specific for P. falciparum heat shock protein-70 (5′-aggtatagaaactgtgggtgg-3′ forward and 5′-gattggttggcatacagcttc-3′ reverse) were used. After PCR amplification, all samples were separated on a 1% agarose gel and stained with ethidium bromide (EtBr) for UV detection. The experiments using oocyst sporozoites and axenic exoerythrocytic stages were done in biological duplicates. The experiments with salivary gland sporozoites, mixed ES, and mixed gametocytes were done in biological triplicates.
Production of recombinant protein for antibody production and purification.
The PFE0565w recombinant protein was produced to use in generating polyclonal antibodies by cloning amino acids 25–212 (eliminating the hydrophobic signal peptide sequence) of the cDNA into the pET32a expression vector containing both thioredoxin and His-tags (Novagen, EMD Chemicals, Madison, WI). The primers used to amplify the region of PFE0565w were 5′-gatcggatccagtgatacctttagagtatttg-3′ forward and 5′-gatcctcgagtcacataaattcctcttgaattttg-3′ reverse. The restriction enzymes, BamHI and XhoI (New England Biolabs, Ipswich, MA), were used for cloning into the pET32a vector. These primers were used to amplify a product by PCR using 2.0 μL DNA (∼100 ng), 1.0 μL FastStart High Fidelity Taq Polymerase (5U/μL, Roche, Indianapolis, IN), 1X FastStart Buffer, 1 mM MgCl2, 0.2 mM di-nucleotide tri-phosphate mix, and 0.5 μM primers. The PCR was conducted using conditions previously described by LaCrue and colleagues.16
The product was double-digested with BamHI and XhoI, along with the pET32a vector, separated by gel electrophoresis, gel-purified according to the manufacturer's instructions using a QIAquick Gel Extraction Kit (Qiagen), and ligated with T4 DNA Ligase (Promega) following the manufacturer's instructions. Two microliters of the ligation product was transformed into DH10B electrocompetant cells by electroporation and streaked on antibiotic-resistant plates. Using colonies that grew on the plates, gDNA was isolated as previously described. The DNA was sequenced at the DNA Core Facility at the University of Missouri and aligned with the PFE0565w sequence available on PlasmoDB using Vector NTI (Invitrogen) to confirm that the correct protein coding sequences were obtained. Once correct sequences/constructs were found, the DNA was transformed into Rosetta-gami DE3 pLysS electrocompetent cells (Novagen). The recombinant proteins were produced by induction with 1.0 mM isopropyl-β-D-thiogalactopyranoside (IPTG) and then purified using a nickel column (Novagen), using the His-tag incorporated into the proteins. Because the purified PFE0565w protein was insoluble, 1 mg of protein was separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the gel stained with Coomassie blue, and the protein excised from the gel for injection into rabbits using a commercial company (Sigma). Two rabbits were injected with a total of 0.7 mg of recombinant protein each. The rabbits were administered a total of six injections containing the protein over a period of 77 days, with the first injection containing 200 μg of protein in complete Freund's adjuvant and the subsequent injections containing 100 μg of protein in incomplete Freund's adjuvant. The PFE0565w preimmune serum was obtained from the rabbits before recombinant protein injection began.
Purification of antibodies.
Pre-absorption of antibodies.
To minimize binding of the PFE0565w antibodies (immune and preimmune) to both mosquito tissues and pET32a bacterial lysate, a pre-absorption protocol, developed by LaCrue and colleagues,16 was conducted as previously described.
Purification of antibodies using pET32a-bound beads.
To further eliminate non-specific binding of anti-PFE0565w antibodies (immune and preimmune) during experiments with the erythrocytic stages and gametocytes, an additional purification protocol was used. Briefly, the pET32a expression vector was induced using 1.0 mM IPTG and the protein lysed with urea buffer (8 M urea, 2 M thiourea, 1% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), and 20 mM DTT). The pET32a protein lysate was then bound to nickel beads (Novagen) following the manufacturer's instructions. The beads were washed twice with 10 mL of urea buffer and washed three times with 10 mL of 1X PBS. The beads were then aliquoted into 1.5 mL centrifuge tubes at a concentration of 50% beads in 1X PBS (200 μL each) and stored at 4°C until use. To purify preimmune and immune PFE0565w serum, a 1:5 dilution (1.0 mL total) of antibodies was made in 1X PBS and added to a tube of pET32a-bound beads, which then rotated for 1 hour at 4°C. After a brief centrifugation, the antibody supernatant was removed and placed into another aliquot of beads to rock one additional hour. Antibodies were then removed from the tube as described previously and aliquoted into clean microcentrifuge tubes for storage at −20°C until use (50 μL each).
Western blot.
The PFE0565w recombinant protein (50 ng, positive control), P. falciparum mixed ES lysate, and mixed gametocyte lysate (10 μL of a 5% parasitemia and 2% gametocytemia, respectively), and non-infected RBCs (10 μL of 50% RBCs, used as a negative control) were resuspended in reducing or non-reducing sample buffer and separated on a 10% gel by SDS-PAGE. The proteins were then transferred to an Optitran nitrocellulose membrane (Sigma) at 15 volts for 50 minutes using a Transblot, SD-semi-dry transfer cell (Bio-Rad, Hercules, CA). The membranes were incubated at RT for 2 hours in blocking solution (5% nonfat, dried milk in 1X Tris-buffered saline, TBS: 50 mM Tris base and 150 mM NaCl, pH 7.4). The membranes were washed three times for 10 minutes with 1X TBS (1X TBS with 0.05% Tween20) and incubated at 4°C overnight in either anti-PFE0565w primary antibody or PFE0565w preimmune serum (1:500 dilution of the antibodies purified with pET32a-bound beads). The next day, the membranes were washed three times for 10 minutes with 1X TBST and then incubated in goat anti-rabbit secondary antibody conjugated with horseradish peroxidase (Cell Signaling Technology, Danvers, MA, 1:10,000 dilution) for 3 hours at RT. Following additional washes, an enhanced chemiluminescent detection method (GE Healthcare, Waukesha, WI) was used, following the manufacturer's instructions, to visualize proteins of interest by autoradiography. Following protein detection, the membranes were stripped in 0.1M NaOH for 5 minutes at RT, washed three times for 10 minutes with 1X TBS, and blocked at 4°C overnight (5% milk in 1X TBS). The membranes were washed as mentioned previously and incubated for 3 hours at RT with either anti-MSP1-19 or anti-Pfs48/45 primary antibodies (1:1,000 dilution), used as a positive control to detect the presence of mixed ES parasites or gametocytes, respectively.18,19 After washing, the membranes were incubated in goat anti-mouse secondary antibody conjugated with horseradish peroxidase (Cell Signaling Technologies, 1:5,000 dilution) for 3 hours at RT. The proteins of interest were then detected by autoradiography. These experiments were done in biological triplicates.
Parasite collection and fixation.
Oocyst and salivary gland sporozoites.
To obtain oocyst sporozoites and salivary gland sporozoites for fixation on slides, midguts and salivary glands were dissected from P. falciparum-infected An. stephensi either 10 days or 14 days PI, counted, and fixed as previously described by LaCrue and colleagues.15 Slides were either used immediately after drying or stored at −20°C until further use.
Mixed erythrocytic stages and Day 16 gametocytes.
Using slides, blood smears of P. falciparum mixed ES parasites (smears containing a mixture of rings, trophozoites, and schizonts) or Day 16 mixed gametocytes (smears containing a mixture of stage I–V gametocytes) were made. The slides were fixed in 100% methanol for 20 minutes at −20°C and in 100% acetone for 5 minutes at RT. Slides were either used immediately after drying or stored at −20°C until further use.
Immunofluorescence assays and confocal microscopy.
Oocyst and salivary gland sporozoites.
Immunofluorescent assays were conducted according to LaCrue and colleagues, except that anti-PFE0565w immune or preimmune antibodies (1:25 dilution using preabsorbed antibodies) were used as one of the primary antibodies along with the anti-circumsporozoite (CS) monoclonal antibody. To visualize the results, either a Radiance 2000 confocal system (Bio-Rad) coupled to an Olympus IX70 inverted microscope (Tokyo, Japan) or a Zeiss LSM 510 Meta NLO confocal microscope (Jena, Germany) was used. The experiments analyzing oocyst sporozoites were done in biological duplicates and the experiments analyzing salivary gland sporozoites were done in biological triplicates.15,16
Mixed erythrocytic stages and Day 16 gametocytes.
The fixed slides, mentioned above, were washed and blocked as previously described for the sporozoite stages. They were then incubated overnight at 4°C with either anti-PFE0565w immune or preimmune antibodies (1:50 dilution using antibodies purified with pET32a-bound beads). The next day, the slides were washed as described previously and incubated for 4 hours at RT with a second primary antibody, anti-MSP1-19 (for mixed ES) or anti-Pfs230 (for gametocytes) antibodies.18,20 The slides were washed and incubated with secondary antibodies as previously described. A nuclear stain, DAPI (Invitrogen), was then added to the slides for 5 minutes at RT. The slides were washed a final time and visualized as mentioned previously for the sporozoite stages. The experiments analyzing mixed ES and mixed gametocytes were each done in biological triplicates.
Creation of a GFP-trafficking construct.
To analyze the protein expression pattern of PFE0565w throughout the life cycle of the parasite and verify other data obtained, a PFE0565w GFP-trafficking construct was made by cloning base pairs 253–1,143 (excluding the stop codon) of the open reading frame of the gene into the pPM2GT vector.21 The primers used to amplify the region were 5′-ccgctcgaggataatatcataaccagaaag-3′ forward and 5′-ccgcctaggcataaattcctcttgaatttg-3′ reverse. The restriction enzymes, XhoI and AvrII (New England Biolabs) were used for cloning into the pPM2GT vector. These primers were used to amplify an 890 bp product using PCR conditions as previously mentioned for generating the recombinant protein construct. The product and vector were double-digested with XhoI and AvrII. Purification, ligation, transformation, and sequencing of the trafficking construct were done as previously described.
Transfection of parasites with the GFP-trafficking construct.
Transfections of P. falciparum were carried out according to Crabb and colleagues.22 Before performing the transfections, mixed ES parasite cultures were synchronized with 5% D-sorbitol (Sigma) for 10 minutes followed by two washes with RPMI 1640 (Invitrogen) at 1,600 × g for 5 minutes 2 days before transfection. In addition, plasmid DNA was isolated using a Plasmid Maxi Kit (Qiagen) and equilibrated in CytoMix (120 mM KCl, 0.15 mM CaCl2, 2 mM EGTA, 5 mM MgCl2, 10 mM K2HPO4 pH 7.6, and 25 mM HEPES pH 7.6). The synchronized P. falciparum ring stage NF54 parasites were electroporated (BTX 600, BTX Harvard Apparatus, Holliston, MA; 0.2 cm cuvette, 0.31 kV, 950 μF, maximum resistance) with 50 μg of the plasmid DNA in CytoMix. Transfected P. falciparum cultures were maintained as previously described.
Two days after electroporation, media containing WR99210 (2.5 nM, Sigma) was added to the cultures to begin the process of selecting transfected parasites using the human dihydrofolate reductase gene drug cassette present in all constructs used.22 To enrich for GFP-trafficking recombinants and eliminate episomal plasmids, parasites were subjected to at least three rounds of drug selection (3 weeks on drug and 3 weeks off drug for each round).
To obtain a clonal population of parasites with the absence of wild-type (WT) parasites carrying episomes, a limiting dilution was performed on the transfected parasites. Parasites were seeded in 96-well plates (200 μL volume) at two concentrations (25% or 50% of wells would contain a single parasite) and maintained in a gassed modular incubator chamber (Billups-Rothenberg, Inc., Del Mar, CA, 3% O2, 3% CO2, and 94% N2) at 37°C. Cultures were gassed every other day for 20 days. On Days 7, 14, and 17, 0.4% fresh RBCs were added. On Day 20, 150 μL of the parasite cultures were transferred to a 96-well plate to begin gDNA isolation for use in PCR and Southern blot analysis to determine if clonal populations of parasites had been successfully created. To isolate the gDNA, 50 μL of 6% saponin (Sigma) was added to the cultures and incubated for 5 minutes at RT. The plate was centrifuged for 15 minutes at 2,650 × g and supernatant removed. One hundred microliters of 1X PBS was added to each well to wash the parasites and the plate was centrifuged again. The 1X PBS was removed and 40 μL of down scale prep buffer (DSP, 1M Tris-Cl pH 8.0, 1M KCl, and 1M MgCl2) working stock (985 μL DSP stock, 10 μL proteinase K, and 5 μL Tween 20) was added to each well and parasite pellets resuspended in the DSP solution.23 The plate was incubated for 30 minutes at 50°C and then for 10 minutes at 95°C. The resulting gDNA was stored at 4°C until further use. The remaining 50 μL of parasites were used for expansion and cryopreservation of promising clonal parasite populations.
PCR and Southern blot verification of the GFP-trafficking construct.
Integration of the transfected DNA at the correct location was verified for each PFE0565w/GFP clone by PCR and Southern blot analysis. To verify integration at the PFE0565w locus by PCR, the primers 5′- atgaagatgattaatattgg-3′ PFE0565w gene-specific forward primer and 5′-tccgtatgttgcatcacc-3′ GFP reverse primer were used in a PCR using 4.0 μL gDNA (isolated from the 96-well plate described previously), 1.25 units GoTaq DNA Polymerase (Promega), 1X GoTaq Flexi Buffer, 1 mM MgCl2, 0.2 mM di-nucleotide tri-phosphate mix, and 0.5 μM primers were used. The PCR conditions used were as previously described, but with an annealing temperature of 52°C. The samples were separated by gel electrophoresis (1% gel) and visualized by UV detection using EtBr.
Southern blotting was performed with gDNA isolated as previously described from ES parasites and digoxigenin (DIG) nonradioactive nucleic acid labeling technology (Roche) was used for visualization of the DNA. Two to three micrograms of gDNA digested with BamHI and BssI were hybridized with an 891-bp fragment of PFE0565w created with the PCR DIG Probe Synthesis Kit following the manufacturer's instructions (Roche). Before hybridization, the DNA was separated on a 0.5% agarose gel and transferred to a positively charged nylon membrane (Osmonics Inc., Minnetonka, MN) overnight by an upward transfer method. Following the manufacturer's instructions, the membrane was washed, hybridized with the above probes, and DNA products detected by autoradiography using the DIG Nucleic Acid Detection Kit (Roche).
GFP-trafficking studies.
The GFP-trafficking studies described below were done in biological duplicates, using two independent PFE0565w/GFP clones obtained by the limiting dilution process described previously.
Mixed erythrocytic stages and gametocytes.
Both P. falciparum mixed ES cultures and Day 16 mixed gametocyte cultures were obtained by collecting 200 μL of infected blood from culture flasks. This protocol was done for all experimental groups: PFE0565w/GFP, NF54 WT negative control parasites, and 3D7HT-GFP positive control parasites.24 The collected, infected blood was centrifuged for 5 minutes at 2,650×g and the supernatant removed. The infected RBCs (iRBCs) were resuspended in 200 μL 1X PBS containing DAPI nuclear stain (1:1,000 dilution, Invitrogen) and incubated in the dark for 5 minutes at RT. The iRBCs were centrifuged again for 5 minutes at 2,650 × g, washed once with 200 μL 1X PBS, centrifuged a final time for 5 minutes, and a small drop of the blood was placed onto a slide. Coverslips were placed on the slides and the slides were viewed with a 100× objective using an Olympus BX51 inverted fluorescent microscope (Center Valley, PA) coupled with an X-Cite Series 120 fluorescent light source. The entire slide was scanned, with at least 100 iRBCs and 50 gametocytes observed for each group.
Zygotes and ookinetes.
For all experimental groups, six midguts were dissected from P. falciparum-infected An. stephensi 24–30 hours PI. The midguts were placed in 1X PBS containing DAPI nuclear stain (1:1,000 dilution) and incubated at RT for 5 minutes. The midguts were then placed three per slide into 15 μL of Matrigel (BD Biosciences, Bedford, MA), coverslips placed on top, and the infected midguts viewed as described previously. Although numbers were limited, at least five zygotes and three ookinetes were observed for each group.
Oocyst sporozoites.
For all experimental groups, six midguts were dissected from P. falciparum-infected An. stephensi 10 days PI. The midguts were placed in 1X PBS containing DAPI nuclear stain (1:1,000 dilution) and incubated at RT for 5 minutes. Three midguts each were placed into 15 μL of 1X PBS on a slide, coverslips placed on the slides, and the infected midguts were examined as described previously. At least half of the midguts had infections with 1–7 oocysts per midgut for each group.
Hemolymph sporozoites.
Hemolymph sporozoites were collected by perfusing the body cavity of 10 P. falciparum-infected An. stephensi 12 days PI with 1X PBS. Hemolymph was collected from all experimental groups by collecting it in microcentrifuge tubes containing 40 μL 1X PBS with DAPI nuclear stain (1:1,000 dilution). The sporozoites were concentrated by centrifugation at 18,000 × g for 5 minutes, supernatant removed, and 10 μL of sporozoites spotted on slides containing 10 μL of Matrigel.16 Coverslips were placed on top of the slides and the slides were viewed as previously described. Two to five hemolymph sporozoites were observed for each group.
Salivary gland sporozoites.
For all three experimental groups, six pairs of salivary glands were dissected from P. falciparum-infected An. stephensi 13–20 days PI. The glands were placed in 1X PBS containing DAPI nuclear stain (1:1,000 dilution) and incubated at RT for 5 minutes. The glands were placed into 15 μL of 1X PBS on a slide, coverslips placed on top, and the infected salivary glands were examined. For each experimental condition, at least 50% of the salivary glands were infected with hundreds of sporozoites observed per set of infected glands.
Results and Discussion
PFE0565w sequence analysis.
PFE0565w is a 1,146 bp gene (containing no intron) located on chromosome five that encodes a 381 amino acid P. falciparum protein with an estimated molecular weight of 45,583 Daltons. Initial PlasmoDB data, based upon mass spectrometry results and sequence analysis, suggested that the PFE0565w protein is expressed in salivary gland sporozoites, has a signal peptide, and one transmembrane domain.10,25 To confirm these data and obtain more information about PFE0565w, additional sequence analysis programs were used. SignalP predicted that PFE0565w has a cleavable signal peptide from amino acids 1–25.26 Analysis using TargetP predicted that the protein enters the secretory pathway and, more specifically, is either a membrane protein located on the surface of the parasite (plasma membrane) or is in the endoplasmic reticulum according to both PSORTII and WoLF PSORT.27,28 Additional sequence analysis using PROSITE, PROTCOMP, Profam, and NCBI (BLASTp) sites predicted the protein has no glycophospholipid (GPI)-anchor, has multiple glycosylation and phosphorylation sites and has no functional identity with other known proteins.29
Next, PlasmoDB indicated that PFE0565w has orthologs in other Plasmodium species. The PFE0565w protein has homology with proteins in Plasmodium vivax (Pv = PVX_080665 in PlasmoDB), Plasmodium knowlesi (Pk = PKH_102160 in PlasmoDB), Plasmodium berghei (Pb = PBANKA_111090 in PlasmoDB), Plasmodium yoelii (Py = PY00913 in PlasmoDB), and Plasmodium chabaudi (Pc = PCHAS_111060 in PlasmoDB). Using a BLAST analysis, the PFE0565w protein does not appear to have any orthologs with other proteins from members outside of the genus Plasmodium (data not shown).14 Even though the proteins listed previously are considered orthologs of PFE0565w, their sequences do not have high homology with PFE0565w (ranging from 15% to 18.1% identity). Like PFE0565w, the proteins listed here are all predicted to have signal peptides and enter the secretory pathway. In addition, the proteins do not have any known function and/or identity with known proteins with the exception of the P. yoelii ortholog, PY00913, which is predicted to be a CCAAT-box DNA binding protein subunit B protein according to data available on PlasmoDB. Therefore, because PFE0565w does not have homology with any known human protein, it could be a good candidate gene for a vaccine.
PFE0565w transcript is present in both mixed ES and mixed gametocyte stages, but its protein is not present in either stage.
The PFE0565w transcript is present in both mixed ES (culture containing a mixture of rings, trophozoites, and schizonts) and mixed gametocyte stages (culture containing a mixture of stage I–V gametocytes), as demonstrated by RT-PCR (1,146 bp product, Figure 1). The gDNA positive control amplified a product and the negative control (no reverse transcriptase) did not. Transcript expression results for PFE0565w obtained during the mixed erythrocytic stages and mixed gametocyte stages are different than data available on PlasmoDB and found while conducting literature searches for PFE0565w and its predicted rodent malaria orthologs in both Plasmodium berghei and Plasmodium yoelii. These data show that its transcript is not present during these stages (free merozoites, rings, trophozoites, schizonts, and gametocytes).30–32 The difference in data obtained from these studies and what is found in the literature may be the result of different experimental techniques used to generate results. All of the data from the literature came from oligonucleotide microarrays and these data were obtained by RT-PCR, where RNA was isolated from large numbers of parasites and used for detecting one gene of interest. If PFE0565w transcripts are present at low levels compared with other genes, they may be undetectable using a microarray approach.
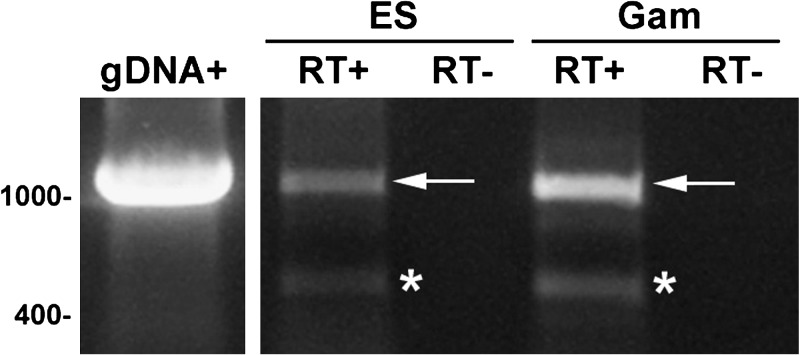
Figure 1.
PFE0565w transcripts are present in the P. falciparum erythrocytic stages, including gametocytes. Primers specific for PFE0565w were used to amplify cDNA products (RT+), indicating the expression of the gene in both mixed erythrocytic stages (ES) and gametocyte stages (Gam) of infection. A 1,146 base pair product was the expected size and was amplified in mixed ES and Gam stages; however there also was an unpredicted product amplified in both the mixed ES and Gam. This product was sequenced and was confirmed to be PFE0565w, missing base pairs 291–988 (the resulting sequence does not translate into a protein). Genomic DNA (gDNA) was used as a positive control and a no reverse-transcriptase (RT–) reaction was used as a negative control to show that the RNA was not contaminated with gDNA. PFE0565w does not contain an intron and the arrow indicates the predicted PFE0565w RT-PCR product and the asterisks indicate the alternative PFE0565w RT-PCR products. This figure is a representative image of three biological replicates of both mixed erythrocytic stages and gametocytes.
Another interesting discovery concerning the transcript presence of PFE0565w during the erythrocytic stages is that a ∼450 bp alternative transcript (see asterisks on Figure 1) is produced during the mixed ES and mixed gametocyte stages. To confirm that this RT-PCR product was PFE0565w, gene-specific primers were used for sequencing the RT-PCR product generated in the PCR analysis. The DNA sequencing using PFE0565w full-length gene-specific primers confirmed it was a 448 bp PFE0565w product, with base pairs 291–988 missing from the sequence (Supplemental Figure 1A). On the basis of the obtained sequence data, this transcript would not be capable of producing a full-length protein, as in silico translation of the sequence using the Translate program from the ExPASy Bioinformatics Resource Portal (www.expasy.org) shows that the truncated transcript does not produce a full length protein (Supplemental Figure 1B). Published data support the hypothesis that this truncated transcript could represent an alternatively spliced gene product. As the transcriptome of P. falciparum continues to be studied, new and/or alternative splice junctions are being discovered and the parasite may need some of these alternatively spliced gene products for either transcriptional or translational regulation.33–36 For example, Sorber and colleagues36 used an in-house developed splice site detection algorithm (HMMSplicer) and found a total of 982 new splice junctions absent from current Plasmodium models. They also found 310 alternative splicing events that occurred in 254 genes during the erythrocytic stages.
Despite the presence of PFE0565w transcript, Western blot analysis showed that the PFE0565w protein (∼43 kDa without a signal peptide) is not present in either mixed ES or mixed gametocytes (Figure 2A). A truncated PFE0565w protein of the size that would represent the alternative PFE0565w transcript product was not observed either (data not shown). Anti-MSP1-19 (19 kDa) or anti-Pfs48/45 (45 kDa) antibodies were used as a positive control to show that parasite protein was present in mixed ES and mixed gametocytes, respectively. The PFE0565w preimmune serum was used as a negative control with no proteins detected in any sample.
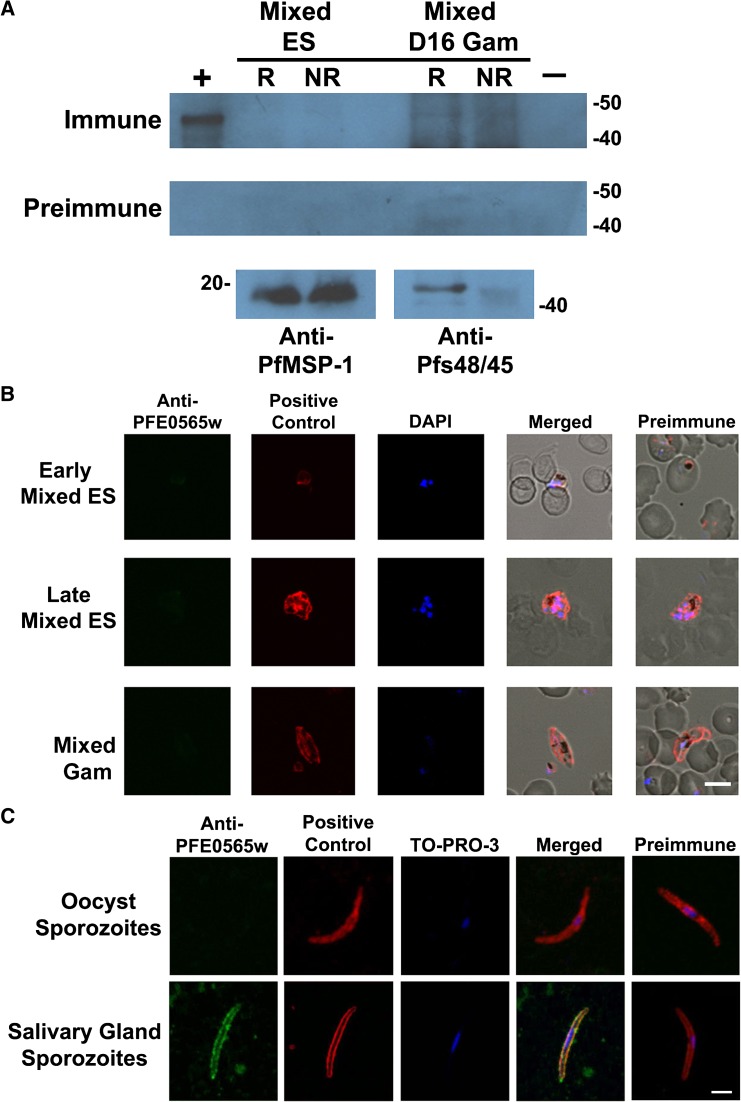
Figure 2.
The PFE0565w protein is not present during the erythrocytic stages or oocyst sporozoites as demonstrated by Western blot analysis and/or confocal microscopy, but is present in salivary gland sporozoites. (A, B) The PFE0565w protein is not present during the erythrocytic stage parasites, including gametocytes, when compared to preimmune controls. PFE0565w would be a 43-kDa product in the Western blot and appear in green in the confocal image. Anti-PfMSP-1-19 and anti-Pfs48/45 or anti-Pfs230 antibodies were used as positive controls for mixed erythrocytic stages (cultures containing a mixture of rings, trophozoites, and schizonts) and mixed gametocytes (cultures containing a mixture of Stage I–V gametocytes at day 16), respectively (red) to demonstrate the presence of parasites. (C) Sporozoites isolated from both mosquito midguts and salivary glands were triple-labeled using anti-PFE0565w antibodies, anti-circumsporozoite (CS) mAb, and TO-PRO-3, a nuclear stain. PFE0565w expression is shown in green and CS, used as a positive control, is shown in red with TO-PRO-3 staining the nucleus blue. The PFE0565w protein is not present in oocyst sporozoites, but is present in salivary gland sporozoites. The merged image for salivary gland sporozoites shows co-localization of PFE0565w with the CS protein, which appears yellow, indicating probable surface expression of PFE0565w. Preimmune serum was used as a negative control for all experiments and no labeling of parasites occurred. This figure is a representative image from three biological replicates for mixed erythrocytic stages, day 16 gametocytes, and salivary gland sporozoites and two biological replicates for oocyst sporozoites. Early = ring or early trophozoite stage parasites; Late = late trophozoite or schizont stage parasites; ES = erythrocytic stages; Gam = gametocytes; R = reduced; NR = non-reduced; + = rPFE0565wB protein (45 kDa, N-terminal portion of protein plus the His-tag, S-tag, and thioredoxin) used as a positive control; and –= non-infected red blood cells used as a negative control. The scale bar in part B represents 5.0 μm and the scale bar in part C represents 2.5 μm.
Results obtained by Western blot were confirmed using immunofluorescent assays. The PFE0565w protein was not detected during mixed ES (11/945 = 1.1%) or gametocyte stages (8/604 = 1.3%, parasites would appear green, Figure 2B and Supplemental Figure 2A and B). Overall, hundreds of parasites were observed (from three biological replicates) on an individual basis, examining multiple parasite stages (rings, trophozoites, schizonts, and stage I–V gametocytes). The labeling of the few parasites that appeared to express PFE0565w was most likely the result of non-specific binding of the anti-PFE0565w antibodies. The PfMSP1-19 and Pfs230 (used as positive controls for mixed ES and gametocytes, respectively) are shown in red, and were expressed by all parasites observed. The DAPI was used as a nuclear stain and appears as blue. The PFE0565w preimmune serum was used as a negative control and no protein labeling occurred. Using data available from PlasmoDB and the literature search described previously, proteomics data acquired during these studies confirm previous findings for PFE0565w, that the protein is not present during erythrocytic stages of the parasite life cycle.10,37–39
PFE0565w transcript is present in both oocyst and salivary gland sporozoites, but the protein is only produced by salivary gland sporozoites.
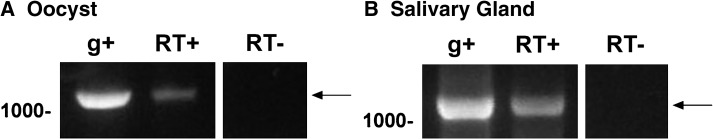
The PFE0565w transcript is present in both oocyst (Figure 3A) and salivary gland (Figure 3B) sporozoite stages, as demonstrated by RT-PCR (1,146 bp transcript). In both stages, the gDNA positive control amplified a product and the negative control (no reverse transcriptase [RT], no RT) did not. According to PlasmoDB and transcript expression studies for PFE0565w in the literature, these data confirm transcript expression in salivary gland sporozoites. The expression of PFE0565w transcript in oocyst sporozoites has not been described elsewhere and represents new knowledge about the P. falciparum transcriptome.
Figure 3.
PFE0565w transcript is present in both the P. falciparum oocyst sporozoite (A) and salivary gland sporozoite (B) stages, as shown by RT-PCR. Primers specific for PFE0565w were used to amplify cDNA fragments of the correct product size (RT+), indicating the expression of the gene during the sporozoite stages of infection. Genomic DNA (g+) was used as a positive control and a no reverse-transcriptase (RT–) reaction was used as a negative control to show that the RNA was not contaminated with genomic DNA. PFE0565w does not contain an intron and the arrows indicate the PFE0565w RT-PCR products. This figure is a representative image from two biological replicates using oocyst sporozoites and three biological replicates using salivary gland sporozoites.
Even though transcript is present in oocyst and salivary gland sporozoites, the PFE0565w protein (visualized in green) is not present during the oocyst sporozoite stage (0/213 = 0.0%); however, it is present during the salivary gland sporozoite stage as demonstrated by immunofluorescent assays coupled with confocal microscopy (507/516 = 98.2%, Figure 2C and Supplemental Figure 2C and D). The CS protein (visualized in red), which is a sporozoite surface protein, was used as a positive control for both oocyst and salivary gland sporozoites and was detected in all parasites (213/213 = 100% for oocyst sporozoites and 516/516 = 100% for salivary gland sporozoites). A merged image is displayed and co-localization of PFE0565w and the CS protein appears as yellow during the salivary gland sporozoite stage, indicating potential surface localization of PFE0565w during the salivary gland sporozoite stage. Preimmune serum was used as a negative control and no protein was detected. Using data available from PlasmoDB and the literature described previously, proteomics data obtained during these studies confirm previous findings for PFE0565w: that the protein is present during the salivary gland sporozoite stage.10,37
Data reveal that PFE0565w transcript is not present during exoerythrocytic stages, suggesting that the PFE0565w protein is not present during exoerythrocytic stages.
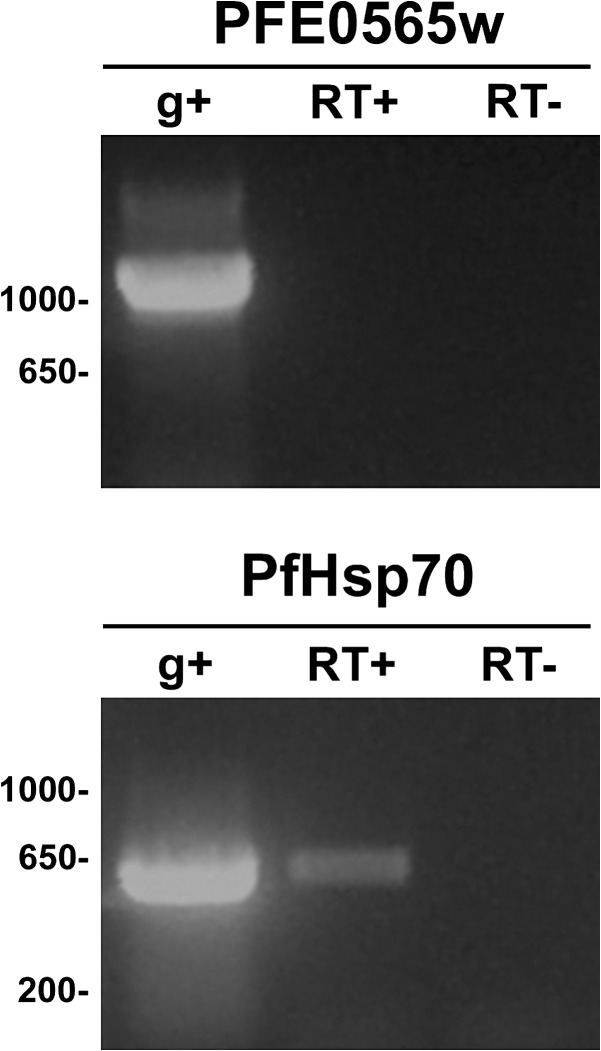
Because the generation of liver stage parasites using primary human hepatocytes was not successful, a method to produce exoerythrocytic stages using axenic cultures (production of liver stage parasites without the presence of liver cells) was used.12 Data obtained from axenic exoerythrocytic stages and RT-PCR suggest that PFE0565w transcript is not present during axenic exoerythrocytic stages (Figure 4). However, a transcript for the PfHsp70 gene, which was used as a positive control because it is highly up-regulated in exoerythrocytic stages, but barely detectable in sporozoites, was amplified, suggesting that liver stage parasites were indeed produced by the axenic cultures. For both PFE0565w and PfHsp70, gDNA was used as a positive control and a no reverse transcriptase reaction was used as a negative control. Exoerythrocytic transcript expression data in the literature for PFE0565w (and its predicted rodent orthologs in P. berghei and P. yoelii) are unresolved. Some literature found expression during these stages, whereas others did not.40–42
Figure 4.
PFE0565w transcript is not present during axenic P. falciparum exoerythrocytic stages as compared to a parasite control transcript that was detected. Primers specific for PFE0565w were used to amplify cDNA fragments (RT+) and no product was amplified, indicating that PFE0565w transcript is not present during the exoerythrocytic stage of infection produced from axenic cultures (i.e., exoerythrocytic parasites generated without the presence of hepatocytes). Primers specific to the P. falciparum heat shock protein-70 (Hsp70) gene were used as a positive control to verify the presence of P. falciparum exoerythrocytic stages. Genomic DNA (g+) was used as a positive control for both primer sets and a no reverse-transcriptase (RT–) reaction was used as a negative control to show that the RNA was not contaminated with genomic DNA.
Because of the difficulty of generating liver stage parasites in vitro, protein expression studies were not able to be conducted for PFE0565w during this particular life cycle stage. Nevertheless, based on initial data suggesting transcript is not present during the exoerythrocytic stages, it is predicted that the PFE0565w protein would not be present during the liver stages.
GFP-trafficking studies confirm that the PFE0565w protein is only present during the salivary gland sporozoite stage.
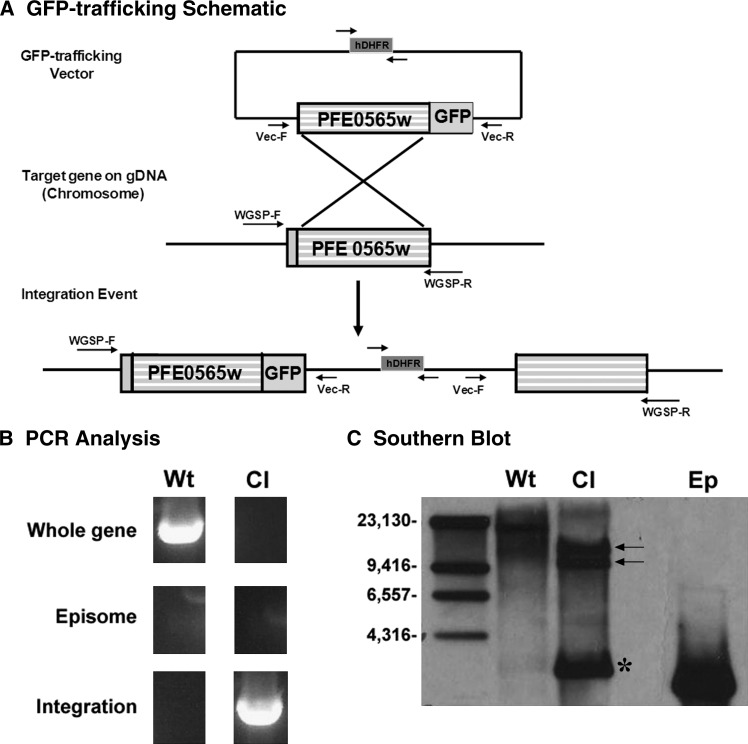
To assess the PFE0565w protein expression profile throughout the entire life cycle of the parasite and confirm data found in the literature, a PFE0565w/GFP trafficking construct was created using the pPM2GT vector and was transfected into the genome of the parasite by a homologous recombination technology (Figure 5A).21 After a limiting dilution, two PFE0565w/GFP clones were isolated after initial PCR analysis (Figure 5B) confirming that they had integrated into the genome of the parasite and that these parasite populations lacked expression of WT parasites carrying episomes. These data were confirmed by Southern blot analysis (Figure 5C), as the predicted integration products of 12,134 bp and 9,762 bp were detected along with no presence of WT parasites carrying episomes. A Southern blot product of unknown origin was also detected and was perhaps caused by a rearrangement of the plasmid that occurred after transfection. This has been previously documented with the pPM2GT vector.21
Figure 5.
A PFE0565w/GFP clonal parasite population was successfully created for trafficking studies throughout the life cycle of the parasite. (A) Transfection schematic demonstrating how the PFE0565w/GFP construct was created using the pPM2GT vector and successfully incorporated into the genome of the parasite via homologous recombination.21 Expression of GFP is driven by the endogenous promoter of PFE0565w. Generation of a PFE0565w/GFP clonal parasite population was verified by both PCR analysis (B) and Southern blot analysis using digoxigenin (DIG) technology coupled with autoradiography (C). Arrows indicate the predicted integration products of 12,134 base pairs and 9,762 base pairs using a PFE0565w specific probe after restriction digestion with BamHI and BssI. The product indicated by an asterisk is of unknown origin and is likely a rearrangement of the plasmid that occurred after transfection. The drug cassette within the vector used for positive selection is human dihydrofolate reductase (hDHFR). This figure is a representative image of the two clonal PFE0565w/GFP parasite populations obtained after limiting dilution. GFP = Green fluorescent protein; Wt = Wild-type parasite genomic DNA; Cl = Clonal PFE0565w/GFP parasite genomic DNA; Ep = PFE0565w/GFP plasmid DNA representing the episome. The arrows on the transfection schematic represent primers used for PCR analysis.
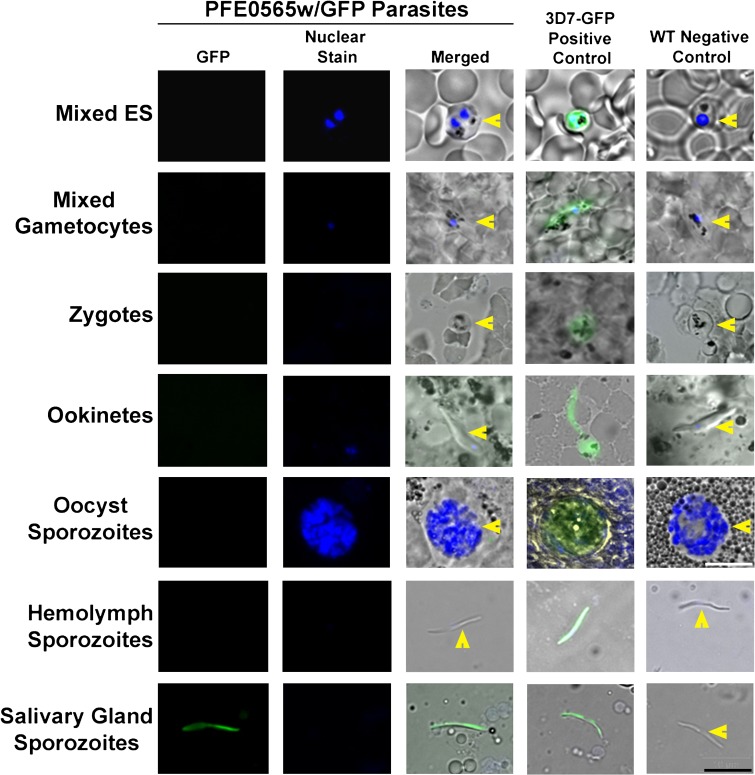
Using the two PFE0565w/GFP clones, two independent GFP-trafficking studies were completed. The WT parasites were used as a negative control and 3D7HT-GFP parasites constitutively expressing GFP throughout the life cycle of the parasite were used as a positive control.24 The various developmental stages of the parasite were examined using fluorescent microscopy (Figure 6). The stages observed were mixed ES (contained a mixture of rings, trophozoites, and schizonts), mixed gametocytes (induced for 16 days and contained a mixture of stages I–V male and female gametocytes), zygotes (24 hours PI), ookinetes (24–30 hours PI), oocyst sporozoites (8–10 days PI), hemolymph sporozoites (12 days PI), and salivary gland sporozoites (13–20 days PI). The PFE0565w protein was not detected in mixed ES, mixed gametocyte stages, zygotes, ookinetes, oocyst sporozoites, and hemolymph sporozoites, despite a reasonable number of parasites being observed for each stage for each replicate. The PFE0565w protein was only detected in salivary gland sporozoites (200/200 = 100% expression in salivary gland sporozoites counted, with hundreds of additional parasites observed as well). For all experiments, all of the 3D7HT-GFP parasites were positive for GFP expression and all of the WT parasites were negative (the parasites did not express GFP). These data confirm previous results obtained by Western blot analysis and by immunofluorescent assays for the mixed ES, mixed gametocyte stages, oocyst sporozoites, and salivary gland sporozoites that the PFE0565w protein is present only during the salivary gland sporozoite stage. In addition, these data confirm previous mass spectrometry results found by Florens and colleagues,10 that the PFE0565w protein is only expressed in salivary gland sporozoites and now provides additional protein expression information (i.e., no expression observed) for P. falciparum stages not previously studied, such as the zygote, ookinetes, and hemolymph sporozoite.
Figure 6.
PFE0565w/GFP-trafficking studies confirm that the PFE0565w protein is only present in salivary gland sporozoites. The PFE0565w protein is not present in the mixed erythrocytic stages (ES, culture representing a mixture of rings, trophozoites, and schizonts), mixed gametocytes (culture representing a mixture of stage I–V gametocytes), zygotes, ookinetes, oocyst sporozoites, and hemolymph sporozoites. The PFE0565w protein is present in salivary gland sporozoites. 3D7HT-GFP (3D7-GFP) constitutively expressing parasites were used as a positive control24 and wild-type (WT) parasites were used as a negative control. This figure is a representative image from two biological replicates (one with each independent clone created). Yellow arrowheads depict the presence of parasites that lack GFP expression (merged images). GFP = green fluorescent protein and nuclear stain = DAPI. The black scale bar in the lower right represents all of the images (except for the oocyst sporozoite stage) and is 10 μm. The white scale bar for the oocysts is 40 μm.
A PFE0565w gene disruption construct was created for functional analysis.
In an attempt to assess the potential function of the PFE0565w protein, gene disruption techniques were used using the pHD22y vector (data not shown).43 After a limiting dilution, one mutant PFE0565w/pHD22y clone was isolated after initial PCR analysis and confirmed by Southern blot analysis (data not shown). Both PFE0565w/pHD22y parasites and WT parasites were maintained in culture and before gametocyte induction, the mutant PFE0565w mixed ES cultures propagated well and appeared to be identical to WT parasite cultures. However, when trying to induce gametocyte formation with the mutant PFE0565w parasites for infection of the Anopheles host, the cultures never produced gametocytes. Instead, these cultures became over-populated with other erythrocytic stages and the parasites died; this occurred during three different attempts when compared with WT controls that did produce gametocytes. Because the PFE0565w protein is not expressed by mixed ES parasites or gametocytes, it is hypothesized that the PFE0565w protein is not essential during this stage of the life cycle. One explanation for the lack of gametocyte development in the mutant PFE0565w/pHD22y parasites is that the parasites were maintained in culture too long (∼5–6 months) before a disruption clone was able to be obtained and the parasites lost their ability to produce gametocytes, which has been previously documented.44,45 The WT control cultures had been maintained in culture for ∼3 months, therefore they may not have lost their ability to produce gametocytes.
In summary, these transcript and protein expression profiles for PFE0565w produced data showing that the protein is only detected in salivary gland sporozoites, despite a broader transcript presence. Because the PFE0565w protein is present in salivary gland sporozoites, the protein may be important for survival/development within mosquito salivary glands and/or may be needed for either development within or invasion of human host tissues. There is evidence in the literature that supports this hypothesis, as PFE0565w has been placed into a category of genes that is highly up-regulated in salivary gland sporozoites compared with other life cycle stages (called a Sporozoite Conserved Orthologous Transcript, SCOT), and their gene products are predicted to be important in the early establishment of exoerythrocytic stages.46 Taken together, PFE0565w could be another candidate for a pre-erythrocytic stage vaccine because it does not share identity with any known human protein.
Supplementary Material
ACKNOWLEDGMENTS
The following reagents were obtained through the MR4 as part of the BEI Resources Repository, NIAID, NIH: Plasmodium falciparum Anti-P30P2-Pf MSP1-19(Q-KNG)FVO-2 rabbit antiserum, MRA-33, deposited by D. C. Kaslow; Mus musculus (B cell); Mus musculus (myeloma) 2A10, MRA-183, deposited by E. Nardin; Mus musculus (B cell); Mus musculus (lymphoma) IIC5B10-1 [2C5B10-1], MRA-316, deposited by L. H. Miller, A. Saul; Pfs230 antibodies (MRA-27) deposited by D. C. Kaslow; Plasmodium falciparum PM2GT (MRA-805) deposited by D. E. Goldberg; P. falciparum 3D7HT-GFP parasites (MRA-1029); Homo sapiens pHD22Y vector (MRA-90) deposited by D. A. Fidock, T. E. Wellems. Finally, the wild-type P. falciparum NF54 parasites were a gift from Shirley Luckhart at the University of California-Davis.
Footnotes
Financial support: This research was supported by the National Institutes of Health/National Institute of Allergy and Infectious Disease (NIH/NIAID) grant, R01AI64306.
Authors' addresses: Maggie S. Schlarman, Renee N. Roberts, Michael M. Kariuki, Ruguang Ou, and Brenda T. Beerntsen, Department of Veterinary Pathobiology, University of Missouri, Columbia, MO, E-mails: magschlarman@gmail.com, rnp975@mail.missouri.edu, kariukim@health.missouri.edu, OuR@missouri.edu, and BeerntsenB@missouri.edu. Alexis N. LaCrue, College of Public Health, Department of Global Health, University of South Florida, Tampa, FL, E-mail: alacrue@health.usf.edu.
Reprint requests: Brenda T. Beerntsen, Department of Veterinary Pathobiology, University of Missouri, 201 Connaway Hall, Columbia, MO 65211, E-mail: beerntsenb@missouri.edu.
References
- 1.Porter WD. Imported malaria and conflict: 50 years of experience in the U.S. Military. Mil Med. 2006;171:925–928. doi: 10.7205/milmed.171.10.925. [DOI] [PubMed] [Google Scholar]
- 2.Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. J Clin Invest. 2010;120:4168–4178. doi: 10.1172/JCI44423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ejigiri I, Sinnis P. Plasmodium sporozoite-host interactions from the dermis to the hepatocyte. Curr Opin Microbiol. 2009;12:401–407. doi: 10.1016/j.mib.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menard R, Sultan AA, Cortes C, Altszuler R, van Dijk MR, Janse CJ, Waters AP, Nussenzweig RS, Nussenzweig V. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature. 1997;385:336–340. doi: 10.1038/385336a0. [DOI] [PubMed] [Google Scholar]
- 5.Sinnis P, Coppi A, Toida T, Toyoda H, Kinoshita-Toyoda A, Xie J, Kemp MM, Linhardt RJ. Mosquito heparan sulfate and its potential role in malaria infection and transmission. J Biol Chem. 2007;282:25376–25384. doi: 10.1074/jbc.M704698200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thathy V, Fujioka H, Gantt S, Nussenzweig R, Nussenzweig V, Menard R. Levels of circumsporozoite protein in the Plasmodium oocyst determine sporozoite morphology. EMBO J. 2002;21:1586–1596. doi: 10.1093/emboj/21.7.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warburg A, Touray M, Krettli AU, Miller LH. Plasmodium gallinaceum: antibodies to circumsporozoite protein prevent sporozoites from invading the salivary glands of Aedes aegypti. Exp Parasitol. 1992;75:303–307. doi: 10.1016/0014-4894(92)90215-v. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, Leach A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum Vaccin. 2010;6:90–96. doi: 10.4161/hv.6.1.9677. [DOI] [PubMed] [Google Scholar]
- 9.Bahl A, Brunk B, Coppel RL, Crabtree J, Diskin SJ, Fraunholz MJ, Grant GR, Gupta D, Huestis RL, Kissinger JC, Labo P, Li L, McWeeney SK, Milgram AJ, Roos DS, Schug J, Stoeckert CJ., Jr PlasmoDB: the Plasmodium genome resource. An integrated database providing tools for accessing, analyzing and mapping expression and sequence data (both finished and unfinished) Nucleic Acids Res. 2002;30:87–90. doi: 10.1093/nar/30.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Florens L, Washburn MP, Raine JD, Anthony RM, Grainger M, Haynes JD, Moch JK, Muster N, Sacci JB, Tabb DL, Witney AA, Wolters D, Wu Y, Gardner MJ, Holder AA, Sinden RE, Yates JR, Carucci DJ. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419:520–526. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 11.Carter R, Ranford-Cartwright L, Alano P. The culture and preparation of gametocytes of Plasmodium falciparum for immunochemical, molecular, and mosquito infectivity studies. Methods Mol Biol. 1993;21:67–88. doi: 10.1385/0-89603-239-6:67. [DOI] [PubMed] [Google Scholar]
- 12.Kappe S, Nussenzweig V, Kaiser K, Camargo N, Singh A. Patent Application. 2010. Plasmodium axenic liver stages as a noninfectious whole organism malaria vaccine. (Patentdocs ed.) [Google Scholar]
- 13.Kissinger JC, Brunk BP, Crabtree J, Fraunholz MJ, Gajria B, Milgram AJ, Pearson DS, Schug J, Bahl A, Diskin SJ, Ginsburg J, Grant GR, Gupta D, Labo P, Li L, Mailman MD, McWeeney SK, Stoeckert CJ, Roos DS. The Plasmodium genome database. Nature. 2002;419:490–492. doi: 10.1038/419490a. [DOI] [PubMed] [Google Scholar]
- 14.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaCrue AN, Sivaguru M, Walter MF, Fidock DA, James AA, Beerntsen BT. A ubiquitous Plasmodium protein displays a unique surface labeling pattern in sporozoites. Mol Biochem Parasitol. 2006;148:199–209. doi: 10.1016/j.molbiopara.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Lacrue AN, James AA, Beerntsen BT. The novel Plasmodium gallinaceum sporozoite protein, Pg93, is preferentially expressed in the nucleus of oocyst sporozoites. Am J Trop Med Hyg. 2005;73:634–643. [PubMed] [Google Scholar]
- 17.Su XZ, Wu Y, Sifri CD, Wellems TE. Reduced extension temperatures required for PCR amplification of extremely A+T-rich DNA. Nucleic Acids Res. 1996;24:1574–1575. doi: 10.1093/nar/24.8.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackman MJ, Whittle H, Holder AA. Processing of the Plasmodium falciparum major merozoite surface protein-1: identification of a 33-kilodalton secondary processing product which is shed prior to erythrocyte invasion. Mol Biochem Parasitol. 1991;49:35–44. doi: 10.1016/0166-6851(91)90128-s. [DOI] [PubMed] [Google Scholar]
- 19.Rener J, Graves PM, Carter R, Williams JL, Burkot TR. Target antigens of transmission-blocking immunity on gametes of Plasmodium falciparum. J Exp Med. 1983;158:976–981. doi: 10.1084/jem.158.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quakyi IA, Carter R, Rener J, Kumar N, Good MF, Miller LH. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol. 1987;139:4213–4217. [PubMed] [Google Scholar]
- 21.Klemba M, Beatty W, Gluzman I, Goldberg DE. Trafficking of plasmepsin II to the food vacuole of the malaria parasite Plasmodium falciparum. J Cell Biol. 2004;164:47–56. doi: 10.1083/jcb200307147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crabb BS, Rug M, Gilberger TW, Thompson JK, Triglia T, Maier AG, Cowman AF. Transfection of the human malaria parasite Plasmodium falciparum. Methods Mol Biol. 2004;270:263–276. doi: 10.1385/1-59259-793-9:263. [DOI] [PubMed] [Google Scholar]
- 23.Adjalley SH, Lee MC, Fidock DA. A method for rapid genetic integration into Plasmodium falciparum utilizing mycobacteriophage Bxb1 integrase. Methods Mol Biol. 2010;634:87–100. doi: 10.1007/978-1-60761-652-8_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talman AM, Blagborough AM, Sinden RE. A Plasmodium falciparum strain expressing GFP throughout the parasite's life-cycle. PLoS ONE. 2010;5:e9156. doi: 10.1371/journal.pone.0009156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bahl A, Brunk B, Crabtree J, Fraunholz MJ, Gajria B, Grant GR, Ginsburg H, Gupta D, Kissinger JC, Labo P, Lil L, Mailman MD, Milgram AJ, Pearson DS, Roos DS, Schug J, Stoeckert CJ, Jr, Whetzel P. PlasmoDB: the Plasmodium genome resource. A database integrating experimental and computational data. Nucleic Acids Res. 2003;31:212–215. doi: 10.1093/nar/gkg081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol. 2000;300:1005–1016. doi: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- 28.Nakai K, Horton P. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci. 1999;24:34–36. doi: 10.1016/s0968-0004(98)01336-x. [DOI] [PubMed] [Google Scholar]
- 29.Gattiker A, Gasteiger E, Bairoch A. ScanProsite: a reference implementation of a PROSITE scanning tool. Appl Bioinformatics. 2002;1:107–108. [PubMed] [Google Scholar]
- 30.Bozdech Z, Llinas M, Pulliam BL, Wong ED, Zhu J, DeRisi JL. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 2003;1:E5. doi: 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, De La Vega P, Holder AA, Batalov S, Carucci DJ, Winzeler EA. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301:1503–1508. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 32.Young JA, Fivelman QL, Blair PL, de la Vega P, Le Roch KG, Zhou Y, Carucci DJ, Baker DA, Winzeler EA. The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol Biochem Parasitol. 2005;143:67–79. doi: 10.1016/j.molbiopara.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Gunasekera AM, Patankar S, Schug J, Eisen G, Kissinger J, Roos D, Wirth DF. Widespread distribution of antisense transcripts in the Plasmodium falciparum genome. Mol Biochem Parasitol. 2004;136:35–42. doi: 10.1016/j.molbiopara.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Hughes KR, Philip N, Lucas Starnes G, Taylor S, Waters AP. From cradle to grave: RNA biology in malaria parasites. Wiley Interdiscip Rev RNA. 2010;1:287–303. doi: 10.1002/wrna.30. [DOI] [PubMed] [Google Scholar]
- 35.Lu F, Jiang H, Ding J, Mu J, Valenzuela JG, Ribeiro JM, Su XZ. cDNA sequences reveal considerable gene prediction inaccuracy in the Plasmodium falciparum genome. BMC Genomics. 2007;8:255. doi: 10.1186/1471-2164-8-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorber K, Dimon MT, DeRisi JL. RNA-Seq analysis of splicing in Plasmodium falciparum uncovers new splice junctions, alternative splicing and splicing of antisense transcripts. Nucleic Acids Res. 2011;39:3820–3835. doi: 10.1093/nar/gkq1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall N, Karras M, Raine JD, Carlton JM, Kooij TW, Berriman M, Florens L, Janssen CS, Pain A, Christophides GK, James K, Rutherford K, Harris B, Harris D, Churcher C, Quail MA, Ormond D, Doggett J, Trueman HE, Mendoza J, Bidwell SL, Rajandream MA, Carcucci DJ, Yates, JR, 3rd, Kafatos FC, Janse CJ, Barrell B, Turner CM, Waters AP, Sinden RE. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005;307:82–86. doi: 10.1126/science.1103717. [DOI] [PubMed] [Google Scholar]
- 38.Khan SM, Franke-Fayard B, Mair GR, Lasonder E, Janse CJ, Mann M, Waters AP. Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell. 2005;121:675–687. doi: 10.1016/j.cell.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 39.Lasonder E, Ishihama Y, Andersen JS, Vermunt AM, Pain A, Sauerwein RW, Eling WM, Hall N, Waters AP, Stunnenberg HG, Mann M. Analysis of the Plasmodium falciparum proteome by high-accuracy mass spectrometry. Nature. 2002;419:537–542. doi: 10.1038/nature01111. [DOI] [PubMed] [Google Scholar]
- 40.Siau A, Silvie O, Franetich JF, Yalaoui S, Marinach C, Hannoun L, van Gemert GJ, Luty AJ, Bischoff E, David PH, Snounou G, Vaquero C, Froissard P, Mazier D. Temperature shift and host cell contact up-regulate sporozoite expression of Plasmodium falciparum genes involved in hepatocyte infection. PLoS Pathog. 2008;4:e1000121. doi: 10.1371/journal.ppat.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarun AS, Peng X, Dumpit RF, Ogata Y, Silva-Rivera H, Camargo N, Daly TM, Bergman LW, Kappe SH. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci USA. 2008;105:305–310. doi: 10.1073/pnas.0710780104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams CT, Azad AF. Transcriptional analysis of the pre-erythrocytic stages of the rodent malaria parasite, Plasmodium yoelii. PLoS ONE. 2010;5:e10267. doi: 10.1371/journal.pone.0010267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fidock DA, Wellems TE. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc Natl Acad Sci USA. 1997;94:10931–10936. doi: 10.1073/pnas.94.20.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schuster FL. Cultivation of Plasmodium spp. Clin Microbiol Rev. 2002;15:355–364. doi: 10.1128/CMR.15.3.355-364.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trager W, Jensen JB. Continuous culture of Plasmodium falciparum: its impact on malaria research. Int J Parasitol. 1997;27:989–1006. doi: 10.1016/s0020-7519(97)00080-5. [DOI] [PubMed] [Google Scholar]
- 46.Westenberger SJ, McClean CM, Chattopadhyay R, Dharia NV, Carlton JM, Barnwell JW, Collins WE, Hoffman SL, Zhou Y, Vinetz JM, Winzeler EA. A systems-based analysis of Plasmodium vivax lifecycle transcription from human to mosquito. PLoS Negl Trop Dis. 2010;4:e653. doi: 10.1371/journal.pntd.0000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.