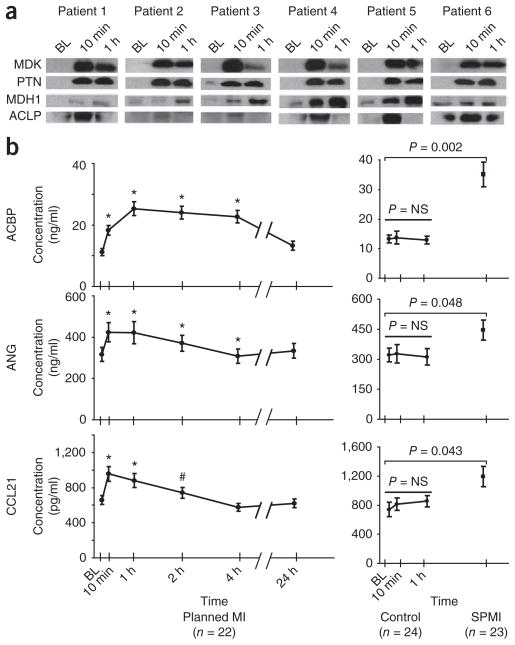
Figure 5.
Verification of candidate biomarkers by western blot analysis and ELISA. (a) Single antibody reagents suitable for western blot analysis were available for MDK, PTN, malate dehydrogenase 1 (MDH1) and aortic carboxypeptidase-like protein 1 (ACLP1). Kinetic analysis of coronary sinus samples from six patients show consistency in the protein changes between the western blot results shown here and the MS-derived temporal trends shown in Figure 3 for the identical proteins. (b) For ANG, ACBP, and C-C motif chemokine 21 (CCL21), sandwiched immunoassays were either constructed (ANG) or commercially available (ACBP and CCL21), and were used to verify protein changes in peripheral plasma from a larger set of PMI patient samples, control samples and spontaneous MI cohorts. In the PMI cohort (b, left) ELISA results confirm significant changes in these candidate biomarkers as early as 10 min after the onset of myocardial injury. In patients with spontaneous MI (b, right) presenting for acute coronary angiography and intervention, significantly higher levels of these proteins were observed as compared to levels in patients who presented to the cardiac catheterization suite with non-acute coronary artery disease (controls, b, center). BL, baseline; NS, not significant; #, P < 0.05; *, P< 0.01.