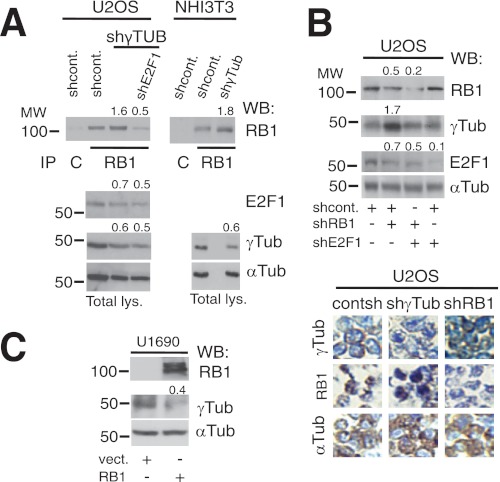
FIGURE 2.
γ-Tubulin and RB1 proteins moderate each other's expression. A, using extracts from U2OS and NIH3T3 cells transfected with control-shRNA (shcont.), E2F1-shRNA (shE2F1), or γ-TUBULIN-shRNA (shγTUB) vectors, RB1 was immunoprecipitated (IP) with a monoclonal anti-RB1 antibody and developed by Western blotting (WB) with a polyclonal anti-RB1 antibody (top). Total lysate (Total lys.) from 1 × 106 of the indicated cell lines was run in parallel and examined by Western blotting using antibodies against E2F1, γ-tubulin, and α-tubulin (Tub) (n = 3). B and C, U2OS or U1690 cells were transfected with the control-shRNA, RB1-shRNA (shRB1), E2F1-shRNA, pSG5 (vect.), or HA-RB1 (RB1) vectors, as indicated; after 48 h (B) or 24 h (C), total lysate from 1 × 106 was prepared and examined by Western blotting using antibodies against RB1, E2F1, γ-tubulin, and α-tubulin (n = 3). B, lower, immunocytochemistry of U2OS cells transfected with γ-TUBULIN-shRNA or RB1-shRNA vectors using the indicated antibodies is shown. A–C, numbers on Western blots indicate variations on protein expression relative to control. To adjust for differences in protein loading, the protein concentration of RB1, E2F1, and γ-tubulin was determined by their ratio with α-tubulin for each treatment. The protein ratio in control treatment was set to 1.