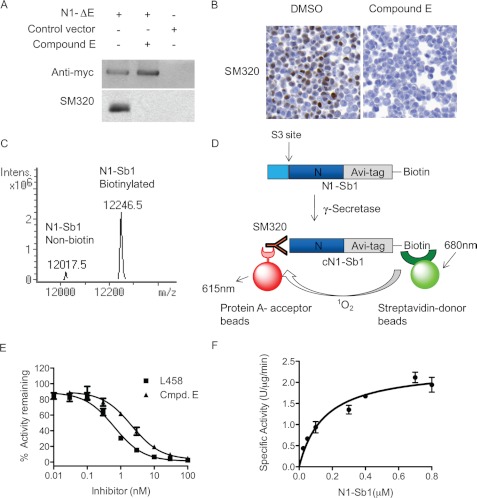
FIGURE 2.
Development of in vitro γ-secretase assay with SM320 antibody and recombinant Notch substrate (N1-Sb1). A, SM320 antibody detects NICD specifically. HEK293 cells were transfected with N1-ΔE, and cell lysates were analyzed with Western blot analysis. Anti-myc antibody detected full-length N1-ΔE in N1-ΔE-transfected cells (top panel, first and second lanes). SM320 antibody recognized NICD in DMSO-treated cells (bottom panel, first lane). This product is absent in the presence of Compound E (bottom panel, second lane). B, immunostaining of HEK293 cells overexpressing N1-ΔE. SM320 antibody detects NICD (brown) in DMSO but not in compound E-treated cells. C, mass spectrometry confirmed that the majority of N1-Sb1 was biotinylated. D, schematic representation of the in vitro γ-secretase assay with N1-Sb1 and SM320 antibody. HeLa cell membrane was incubated with N1-Sb1 in the presence of 0.25% CHAPSO. The C-terminal of cleaved N1-Sb1 (cN1-Sb1) was detected with SM320 antibody. Streptavidin-conjugated donor beads and protein A-conjugated acceptors beads were added to the reaction to detect cN1-Sb1. E, potencies of γ-secretase inhibitors L458 and compound E were determined to be 0.7 nm and 1 nm, respectively. F, the kinetic analysis of N1-Sb1 proteolysis. The specific activity was expressed as the arbitrary AlphaLISA assay unit.