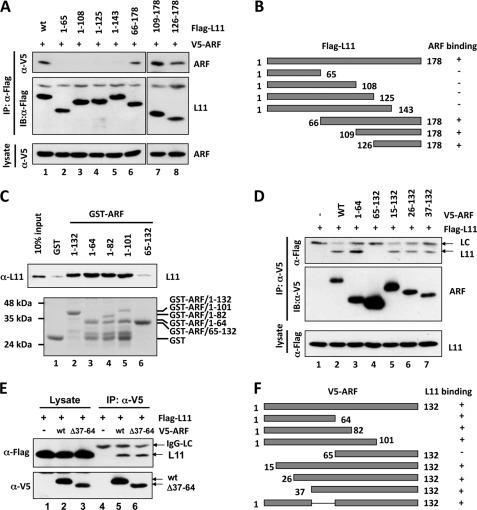
FIGURE 3.
Mapping the binding domains between ARF and L11. A, ARF binds to the C-terminal domain of L11 in cells. H1299 cells were transfected with V5-ARF and Flag-tagged wild-type (wt) L11 or its deletion mutants as indicated. The cell lysates were immunoprecipitated with the anti-Flag antibody followed by IB with anti-V5 or anti-Flag antibodies (top panels). B, diagram of the Flag-L11 and its deletion mutants. The co-IP results determined in panel A are shown on the right. “+” indicates binding and “−” indicates lack of binding. C, L11 binds to the N-terminal domain of ARF in vitro. Bacterially purified GST, GST-ARF, or their deletion mutants immobilized on glutathione beads, were incubated with bacterially expressed His-L11. Bound L11 was detected using IB with anti-L11 antibody (top panel). The purified GST and GST-ARF proteins were shown in the bottom panel by Coomassie Blue staining. D and E, L11 interacts with the N-terminal domain of ARF in cells. H1299 cells were transfected with Flag-L11 together with V5-ARF, or its deletion mutants, as indicated. The cell lysates were immunoprecipitated with the anti-V5 antibody followed by IB with anti-V5 or anti-Flag antibodies. F, diagram of V5-ARF and its deletion mutants used in D and E. The co-IP results determined in panels D and E are shown on the right. “+” indicates binding and “−” indicates lack of binding.