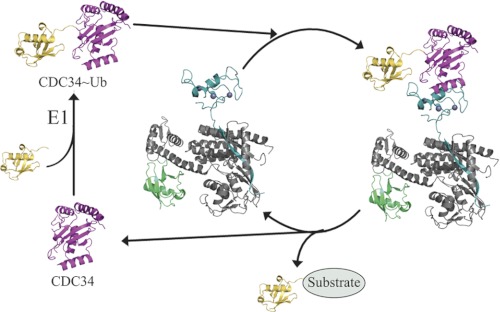
FIGURE 7.
Rbx1/ROC1-CDC34∼ubiquitin (Ub) ternary complex controls polyubiquitylation. Structures shown represent the catalytic domain of CDC34 (pink), ubiquitin (yellow), Rbx1/ROC1 (teal), the C-terminal domain of CUL1 (gray), and NEDD8 (green). CDC34∼Ub was modeled by aligning CDC34 (PDB 2OB4) with the Ubc13∼Ub structure (PDB 2GMI) (65). The Rbx1/ROC1-CUL1-NEDD8 structure was derived from PDB 3DQV (CUL5) (9). After the charging of CDC34 with ubiquitin, CDC34∼ubiquitin binds to Rbx1/ROC1, forming the ternary Rbx1/ROC1-CDC34∼ubiquitin complex. Upon ubiquitin transfer to a substrate protein, the spent CDC34 is released from Rbx1/ROC1. This release may allow another CDC34∼ubiquitin to bind to Rbx1/ROC1 for the next round of ubiquitin transfer. Alternatively, CDC34 may remain associated with CUL1 via its extended C terminus (not shown) proposed to bind with a basic canyon on the lower side of CUL1. In either case CDC34 must be recharged with ubiquitin by the E1 enzyme before it can re-engage with Rbx1/ROC1 for subsequent ubiquitin transfer.