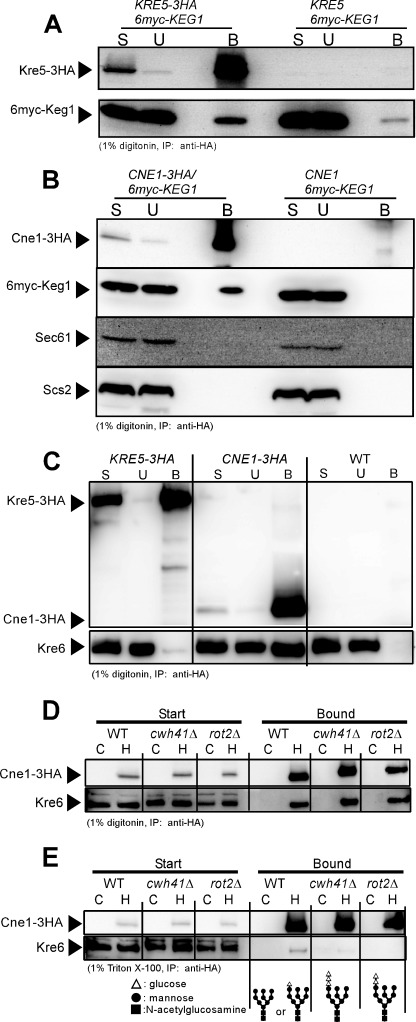
FIGURE 6.
Protein-protein interactions between calnexin cycle member homologues and Keg1 or Kre6. A, immunoblots of the start (S), unbound (U), and bound (B) materials of the immunoprecipitation experiment using anti-HA monoclonal antibody from the cleared cell lysates containing 1% digitonin are shown. Kre5-3HA and 6myc-Keg1 were detected by anti-HA and anti-myc monoclonal antibodies, respectively. Materials derived from 40-fold more cells were loaded for the B sample than for the others in SDS-PAGE. B, similar experiments to A were done to see the interaction between Cne1-3HA and 6myc-Keg1. As Cne1 may be a chaperon, ER membrane proteins Sec61 and Scs2 were also analyzed as the controls. Materials derived from 80-fold more cells were loaded for the B sample than for the others in SDS-PAGE. C, interaction between Kre6 and Kre5-3HA or Cne1-3HA was examined, as in A. Kre6 was detected by anti-Kre6 antiserum. Materials derived from 50-fold more cells were loaded for the B sample than for the others in SDS-PAGE. D, interaction between Cne1-3HA and Kre6 was examined by immunoprecipitation from the cleared cell lysate of calnexin cycle mutants in the presence of 1% digitonin. Lanes C and H indicate that the cell lysates were prepared from the wild-type CNE1 and HA-tagged CNE1-3HA strains, respectively. Materials derived from 30-fold more cells were loaded for the Bound sample than for the Start sample in SDS-PAGE. E, similar experiments to D were done by replacing digitonin with Triton X-100. Materials derived from 190-fold more cells were loaded for the Bound sample than for the Start sample in SDS-PAGE. The expected molecular structures of N-glycan in the strains used are shown at the bottom.