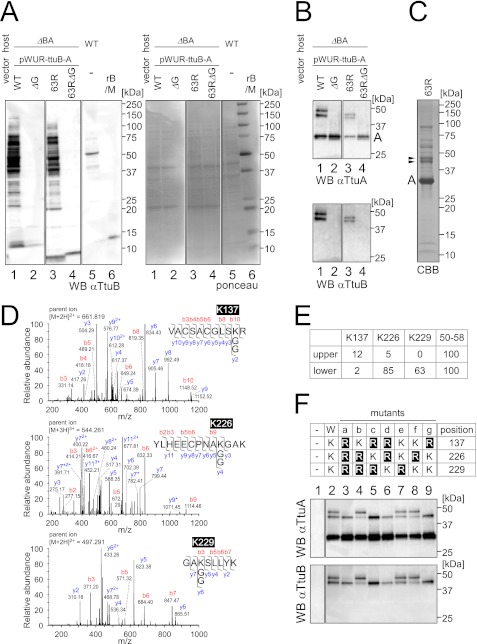
FIGURE 4.
Identification of the attachment sites of TtuA-TtuB conjugates. A, left panel, immunoblotting of extracts (22 μg) from the ΔttuB-ttuA strain transformed with pWUR-ttuB-ttuA against an anti-TtuB antibody. TtuB (wild type (lane 1), C-terminal glycine deletion mutant (lane 2), S63R mutant (lane 3), or S63R with C-terminal deletion mutant (lane 4)) was coexpressed with wild-type TtuA. For comparison, an extract (22 μg) from the wild-type strain was also analyzed (lane 5). Recombinant TtuB (rB) (6 ng) and molecular weight marker (M) were loaded in lane 6. Right panel, the Ponceau-stained membrane shows equal amounts of protein loaded. B, cell extracts (same as in A) were immunoprecipitated with an anti-TtuA antibody, and the eluates were analyzed by Western blot analysis. The membrane was probed with anti-TtuA (upper panel) and reprobed with anti-TtuB (lower panel). C, large-scale immunoprecipitation from ΔttuB-ttuA strain with the expression plasmid for TtuA and TtuB (S63R). The eluate was resolved by SDS-PAGE, and proteins were visualized using Coomassie Brilliant Blue R-250 (CBB). The bands corresponding to TtuA-TtuB conjugates (shown by arrowheads) were excised and subjected to MS analysis. D, lysine 137, lysine 226, and lysine 229 in TtuA were identified as TtuB attachment sites by LC-MS/MS analysis. Three peptides contained a lysine residue modified with two glycines. The collision-induced dissociation spectrum of Lys-137 peptide obtained from the upper band in C is shown in upper panel. For the Lys-226 peptide (center panel) and the Lys-229 peptide (lower panel), the spectra obtained from the lower band are shown. Peptide fragments were identified as b- or y-type ions and deaminated ions (asterisk). E, estimation of the abundance of the modified peptides of TtuA (upper and lower bands in C) by MS analysis. The peptides were roughly quantified by the parent ion mass chromatograms. The values were normalized by using a peptide corresponding to positions 50–58 of TtuA in each band. F, mutational analysis of the identified lysines to arginines in TtuA. The wild type and seven types of mutants (a–g) of TtuA (upper panel) were coexpressed with wild-type TtuB in the T. thermophilus ΔttuB-ttuA strain. The strain with the empty vector was also analyzed (- in lane 1). Cell extracts were immunoprecipitated with an anti-TtuA antibody, and the eluates were analyzed by Western blot analysis. The membrane was probed with anti-TtuA (center panel) and reprobed with anti-TtuB (lower panel).