FIGURE 6.
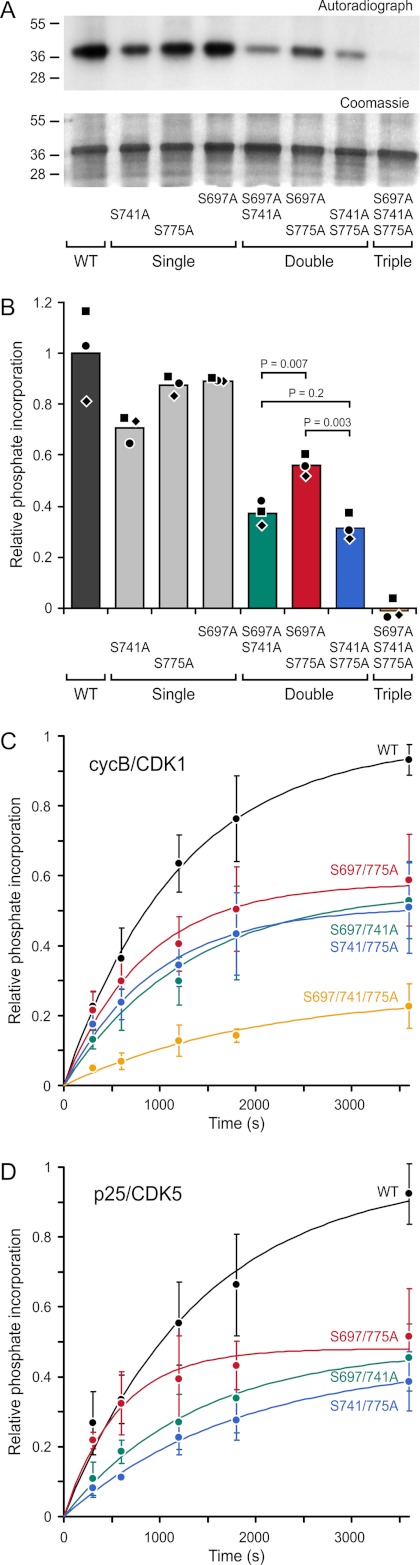
Mitotic and interphase CDKs phosphorylate priming sites in the CLASP2 GSK3 motifs. A, shown is in vitro phosphorylation of CLASP2-(497–794) and the indicated phosphorylation site mutations by cycB/CDK1. Top panel, autoradiograph; bottom panel, corresponding Coomassie-stained gel. Sizes of molecular mass markers (kDa) are indicated on the left. B, shown is quantification of phosphate incorporation from three independent experiments (indicated by different shaped symbols) normalized to the average wild-type value. Three serine residues are phosphorylated by cycB/CDK1 in the CLASP2 plus-end-tracking domain, and Ser-741 appears to be the preferred site. C and D, shown are progress curves of phosphorylation of the indicated double priming site mutant constructs by cycB/CDK1 (n = 4) and p25/CDK5 (n = 3). Data are normalized to the wild-type protein in each experiment. Error bars indicate 95% confidence intervals, and the progress curves were approximated by an exponential fit (solid lines). As judged by the initial rates, p25/CDK5 appears to prefer Ser-741 even more than cycB/CDK1.