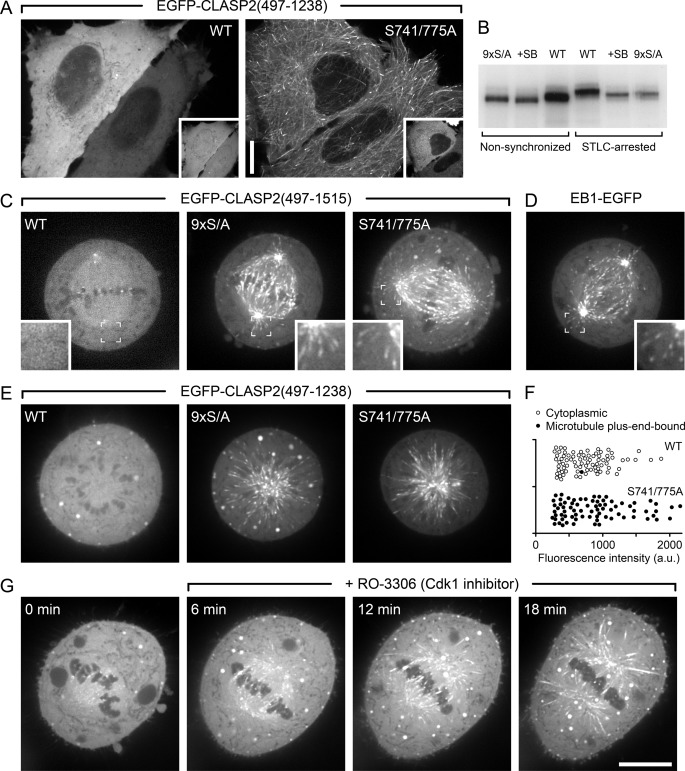
FIGURE 8.
CLASP2 plus-end-tracking is inhibited during mitosis. A, shown are interphase cells expressing either wild-type EGFP-CLASP2-(497–1238) or the double-priming site mutant (S741A/S775A) together with constitutively active mRFP-GSK3β(S9A). Insets indicate GSK3β expression. B, shown is metabolic labeling of HeLa cells with 32P-labeled phosphate. EGFP-CLASP2-(497–1238) was immunoprecipitated and analyzed by SDS-PAGE and autoradiography. Note that radioactivity incorporation is not directly comparable between non-synchronized and STLC-arrested cells because of differences in loading due to limited recovery of mitotic cells. However, the increased gel shift in STLC-arrested cells indicates increased mitotic phosphorylation. In addition, decreased radioactivity incorporation and downshift in the presence of a GSK3 inhibitor (20 μm SB216763) or of the non-phosphorylatable mutant (9× S/A) demonstrates GSK3-mediated phosphorylation in both interphase and mitosis. C, shown are HeLa cells in metaphase expressing wild-type (WT) EGFP-CLASP2-(497–1515) or constructs in which all GSK3 sites (9× S/A) or only SXIP motif-associated CDK priming sites (S741A/S775A) were mutated. Insets show the indicated regions at higher magnification. D, shown is metaphase cell-expressing EB1-EGFP. E, HeLa cells expressing the indicated EGFP-CLASP2-(497–1238) constructs that lack the kinetochore binding domain were treated with STLC, which results in monopolar spindles arrested in a metaphase-like state. F, quantification of data in E demonstrates that mutation of the priming sites alone restores CLASP2 plus-end-tracking during mitosis. Each symbol represents the average EGFP-CLASP2-(497–1238) fluorescence intensity in the cytoplasm of one cell (n = 80). a.u., arbitrary units. G, EGFP-CLASP2-(497–1515)-expressing HeLa cells arrested in metaphase with MG132 and subsequently treated with the CDK1 inhibitor RO-3306 results in the reappearance of EGFP-CLASP2-(497–1515) on microtubules. Scale bars, 10 μm.