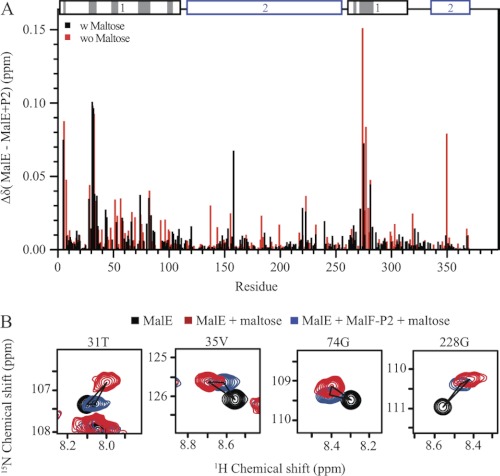
FIGURE 2.
Chemical shift titration experiments of MalE and MalF-P2 in the presence and absence of maltose. A, resonance-specific 1H-15N chemical shift changes upon titration of MalF-P2 to U-15N,2H MalE, in the presence (black) and absence (red) of maltose. Two distinct binding regions in the N-terminal domain of MalE are observed. These regions match the contacts seen in the crystal structure of MalFGK2-E (PDB ID code 2R6G) (6). MalE and MalF-P2 contacts (with Δ(N, C, O) <5 Å) in the MalFGK2-E crystal structure are highlighted in gray on the primary sequence (top). In addition, small chemical shift changes are observed for residues not directly involved in the interaction. B, 1H,15N correlation spectra for selected residues, not located in the binding interface. Resonances are color coded, MalE (black), MalE/maltose (blue), and MalE/MalF-P2/maltose (red). Chemical shift analysis shows resonances distinct from the maltose-bound and maltose-free MalE, implying that MalE in the presence of MalF-P2 adopts a different conformation.