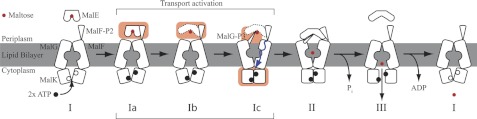
FIGURE 9.
Activation model of substrate transport in MalFGK2. Based on the alternative-access model (Fig. 1), three additional intermediates are proposed to account for our findings. Ia, MalK subunits in an open state containing bound ATP. Substrate recognition is mediated through binding of MalF-P2. Ib, conformational change of MalE, exposing the substrate to the solvent and allowing insertion of MalG-P3 into the binding pocket (dashed lines MalE). Ic, substrate availability signaled reciprocally across the membrane inducing a closure of the MalK dimer. Subsequently, the transmembrane MalF/G domains switch from an inward-facing to an outward-facing conformation allowing the released maltose to bind into the binding pocket of the transmembrane domains of MalF/G. Amplitudes of motion for the TMDs are exaggerated to highlight changes. A TMD rotation from the inward to the outward conformation was determined to be on the order of 22 ° (4).