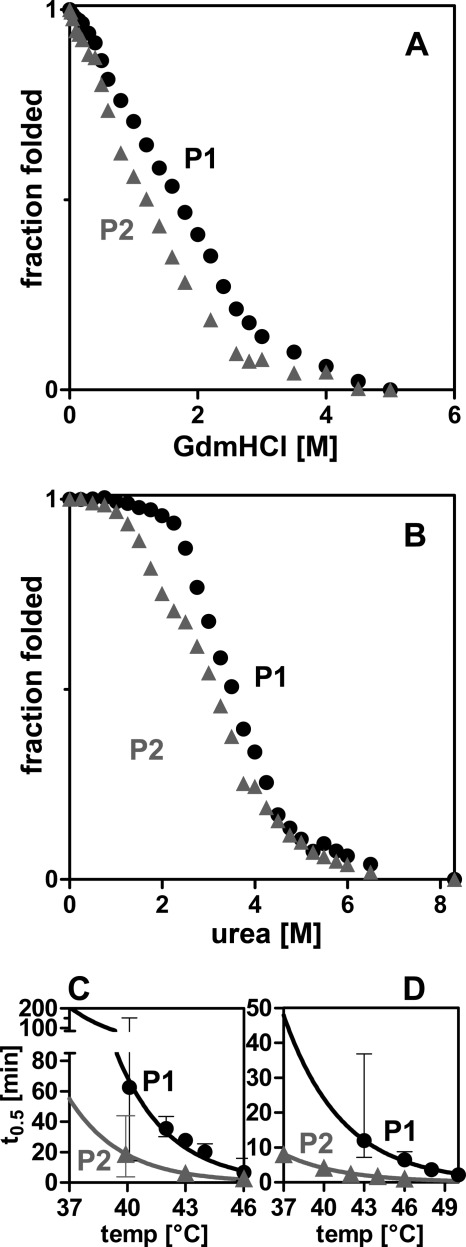
FIGURE 1.
PAPS synthases differ in their protein stability. A and B, chemical unfolding of human PAPS synthases by guanidine hydrochloride (GuaHCl) and urea monitored by intrinsic tryptophan fluorescence. Irreversible unfolding proceeds via at least one intermediate. Midpoints of unfolding were at 1.70 and 1.19 m GdnHCl, as well as 3.50 and 3.11 m urea for PAPS synthases 1 and 2 (P1 and P2), respectively. C and D, loss-of-activity measurements of APS kinase and ATP sulfurylase domains, respectively. Proteins were incubated for increasing times at elevated temperatures. Subsequently, residual activity was measured under standard conditions. Error bars represent 95% confidence intervals.