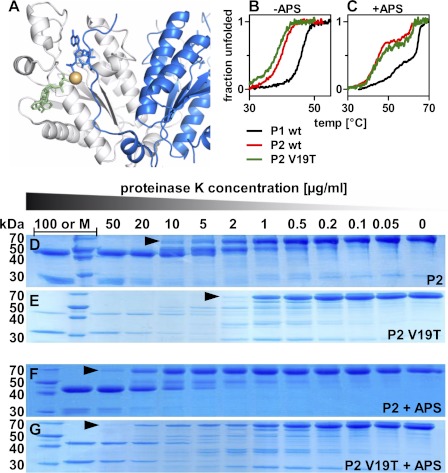
FIGURE 7.
The V19T mutant of PAPSS2. A, highlighting Thr-29 within the crystal structure PDB code 2OFX of the kinase domain of human PAPSS1. This position of this residue is occupied by valine in PAPSS2. It was also highlighted by random forest analysis. B, unfolding transition for the V19T mutant of PAPSS2. The midpoint of unfolding is lowered from 39.8 to 38.2 °C. C, in presence of APS, however, unfolding is more than rescued. There, the midpoint of the unfolding transition is even shifted to higher temperatures from 45.3 to 47.0 °C indicative of a specific role of threonine at this sequence position. D and E, proteolysis of PAPSS2 by limiting amounts of proteinase K (PK). The amount of PK needed to cleave the full-length proteins differs more than 5-fold for wild-type and V19T mutant proteins. F and G, addition of 50 μm APS stabilized the V19T mutant protein nearly to the wild-type level.