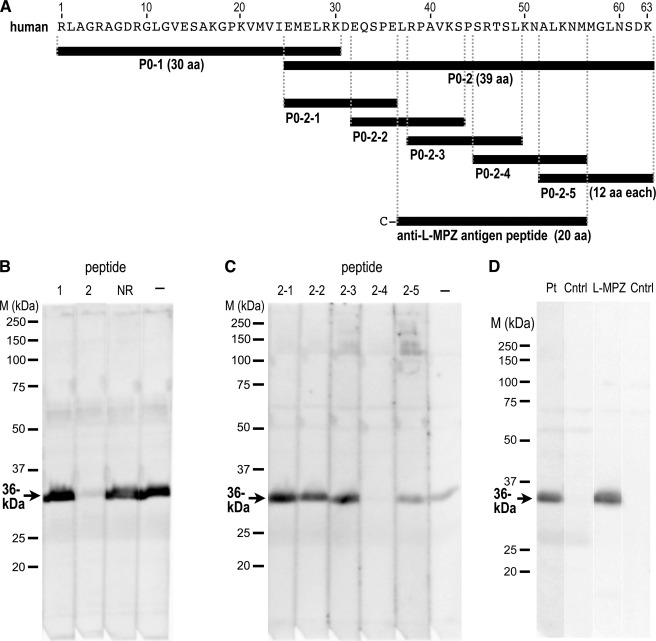
FIGURE 4.
Elimination of immunoreactivity for the 36-kDa band by specific peptides. A, black bars depict the synthesized peptides with the additional 63-aa sequence: two peptides derived from the 63-aa sequence (P0–1 and P0–2), five partially overlapping peptides derived from P0–2 (P0–2-1 to -5), and a peptide used to produce the specific antibody in rabbit. A cysteine residue was added to the N terminus of the latter peptide for conjugation to keyhole limpet hemocyanin. B and C, peptide absorption tests of the 36-kDa protein with immunoblots using serum IgG from a patient with CIDP. The immunoreactivity was completely blocked by the P0–2 peptide but not by P0–1 or a control non-related 21-aa peptide (NR; B). Only P0–2-4 absorbed the immunoreactivity of the 36-kDa band; P0–2-1, -2, -3, and -5 did not (C). The positive control (−) consisted of a sample containing no peptide. D, Western blotting using rabbit anti-L-MPZ produced by immunization with the synthetic peptide shown in A. The same 36-kDa band was recognized by the patient serum (Pt) and anti-L-MPZ (L-MPZ). The negative control (Cntrl) in each case had no primary antibody. Western blots of ScN whole homogenate had 10 μg protein/lane (B and C) or 5 μg protein/lane (D). M, marker.