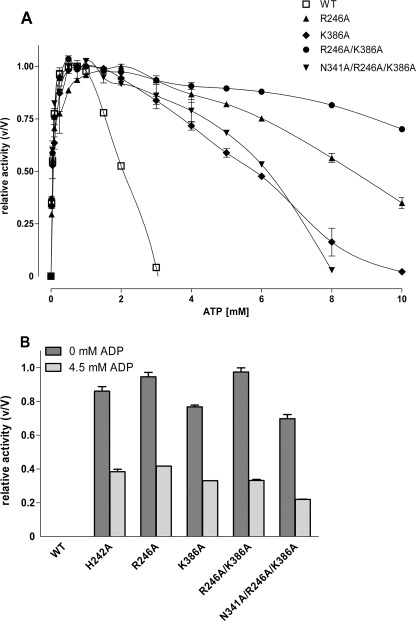
FIGURE 4.
Effect of ATP and ADP on activity of wild type Pfk and various mutations at the inhibitory allosteric site. A, the effect on the Pfk activity of increasing concentrations of ATP at the wild type, R246A, K386A, R246A/K386A, and the enzyme mutated additionally at the activating site (N341A/R246A/K386A) was determined at 2 mm Fru-6-P. Activity is expressed relative to maximal activity for each enzyme under these conditions (V values were 274, 233, 213, and 164 units/ml for R246A, K386A, R246A/K386A, and N341A/R246A/K386A, respectively). To compare data with N341A, see Fig. 2C. B, the activity of wild type and the mutants was measured at 0.5 mm Fru-6-P and 1 mm ATP in the absence and presence of 4.5 mm ADP (inhibitory concentration). Under both conditions, the wild type and N341A displayed no significant activity. Activity is expressed relative to maximal activity (V) for each enzyme under these conditions (V values: wild type, 295 units/ml; H242A, 270 units/ml; R246A, 237 units/ml; K386A, 171 units/ml, R246A/R386A, 250 units/ml; N341A/R246A/K386A, 209 units/ml). Data are means ± S.E. of three independent experiments each performed in duplicate.