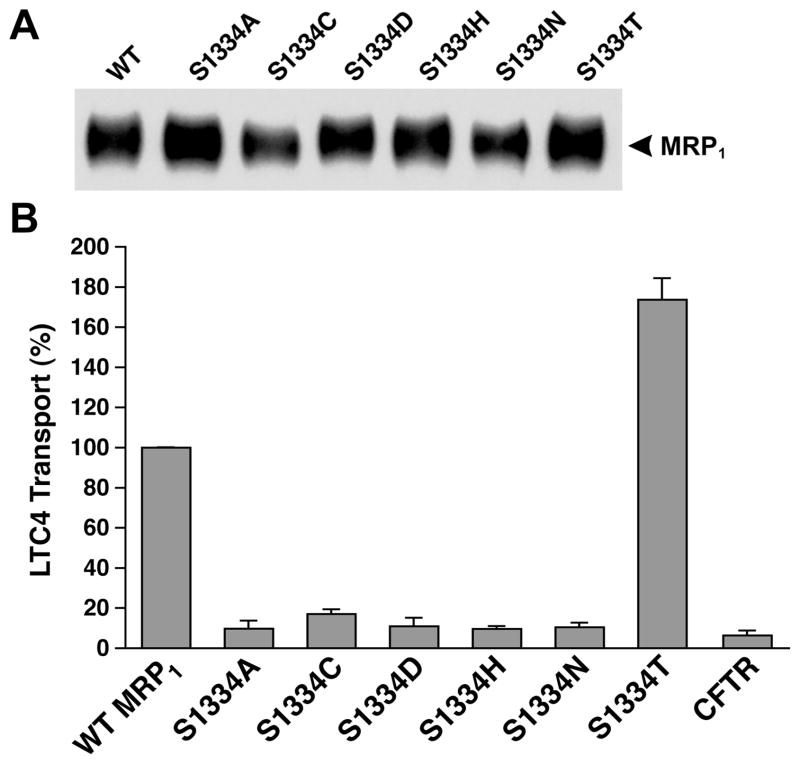
Figure 2.
Substitution of S1334 with an amino acid that eliminates the hydroxyl group abolishes the ATP-dependent LTC4 transport. (A) MRP1 contents in membrane vesicles. Membrane vesicles were prepared according to the method described in Experimental Procedures. The amounts of proteins loaded in the gel are 400 and 800 ng (only the results derived from 800 ng of protein are shown). MRP1 on the right indicates the complex-glycosylated mature MRP1 that was detected in Western blot by employing the monoclonal antibody 42.4 against NBD1 (8). The intensities of the MRP1 bands were determined by a scanning densitometer. The mean ratios (n = 2, including the results derived from 400 and 800 ng of protein), considering the amount of wild-type MRP1 as 1, of the mutant proteins are as follows: S1334A, 1.46 ± 0.06; S1334C, 0.84 ± 0.14; S1334D, 1.07 ± 0.69; S1334H, 1.17 ± 0.16; S1334N, 0.97 ± 0.08; S1334T, 1.30 ± 0.11. (B) ATP-dependent LTC4 uptake by membrane vesicles prepared from the cells expressing either wild-type or mutant MRP1s. The assays were carried out in a 30 μL solution containing 3 μg of membrane vesicles (the amount of MRP1 protein determined in panel A was adjusted to a similar amount by adding a varying amount of membrane vesicles prepared from parental BHK cells) and 4 mM AMP (used as a control) or 4 mM ATP at 37 °C for 1 min. The amount of LTC4 bound to the membrane vesicles in the presence of 4 mM AMP was subtracted from the corresponding samples in the presence of 4 mM ATP. The data are the means ± SD of three triplicate determinations.