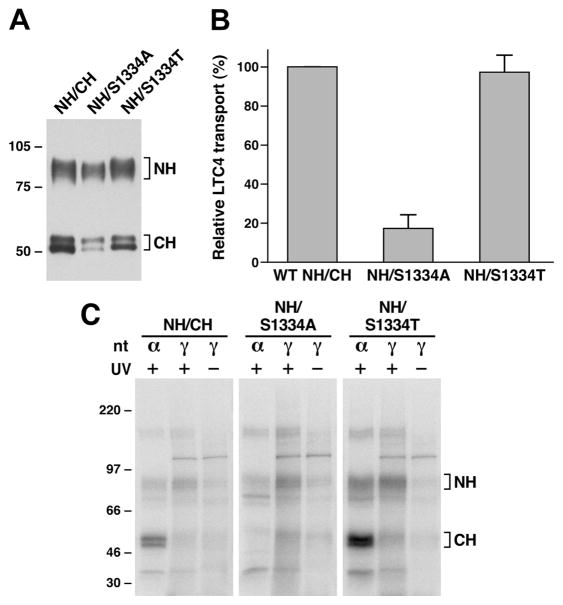
Figure 5.
Substitution of S1334 with an alanine residue significantly impaired ATP binding/hydrolysis at the mutated NBD2. (A) MRP1 contents in membrane vesicles prepared from Sf21 cells. Membrane vesicles were prepared according to the method described in Experimental Procedures. The amounts of proteins loaded in the gel are 300 ng of membrane proteins. NH and CH on the right indicate the N-terminal half (detected by the mAb 42.4 against NBD1) and C-terminal half (detected by the mAb 897.2 against NBD2). The intensities of the MRP1 bands were determined by a scanning densitometer. The mean ratios (n = 2, including the results derived from 200 and 300 ng of protein), considering the amount of wild type MRP1 as 1, of the mutant proteins are as follows: S1334A, 0.68 ± 0.06; S1334T, 0.90 ± 0.06. (B) ATP-dependent LTC4 uptake by membrane vesicles prepared from wild type and mutant MRP1s. The assays were carried as described in Figure 2B. (C) S1334A-mutated MRP1 affects both ATP binding and hydrolysis at the mutated NBD2. Wild type MRP1 or S1334A- or S1334T-mutated MRP1 was introduced into the pDual/N-half/C-half and expressed in Sf21 insect cells (12, 25). The membrane vesicles prepared from the viral particle infected Sf21 cells were used to do photolabeling as described in Figure 4. NH and CH on the right indicate the 32P-nucleotide-labeled NBD1-containing N-half and NBD2-containing C-half.