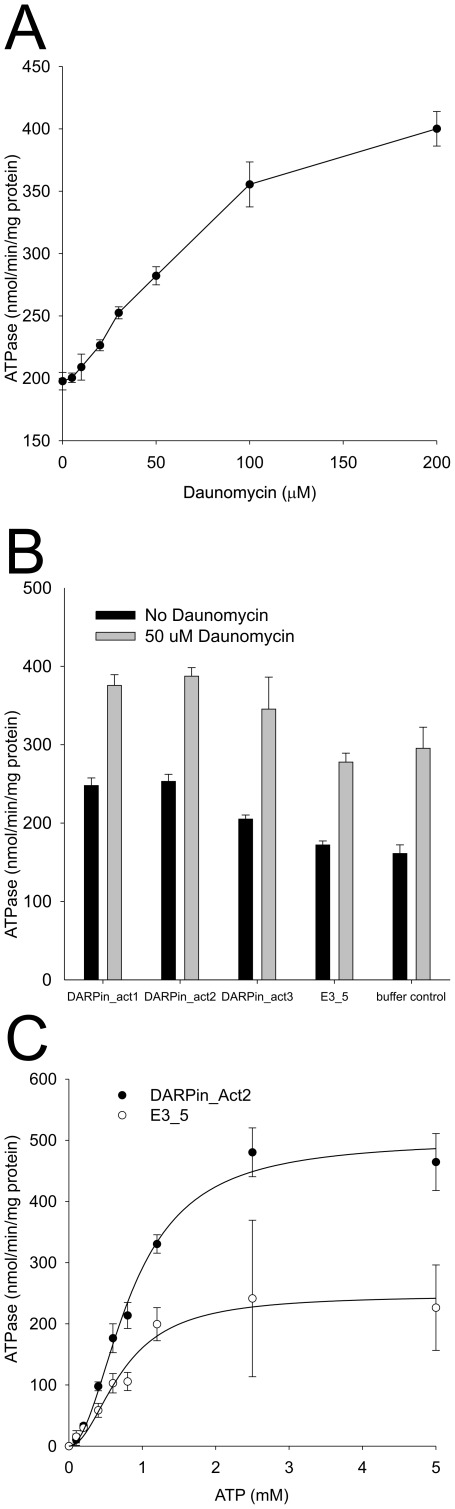
Figure 9. ATPase activity of reconstituted LmrCD is stimulated by DARPin activators and daunomycin.
Each symbol or bar represents the average of three data points. (A) The ATPase activity of reconstituted LmrCD is stimulated in the presence of daunomycin in a dose-dependent manner. (B) Reconstituted LmrCD (protein:lipid ratio of 1∶50, proteoliposomes diluted to obtain an LmrCD concentration of 70 nM) was incubated with DARPin activators and control DARPin E3_5* (2.5 µM) and the ATPase activity was determined in the absence and presence of 50 µM daunomycin (triplicates). As a control, buffer instead of DARPins were added to LmrCD. According to t-test analysis, the measured ATPase activity differences between DARPin_Act1 to Act3 and the buffer control are statistically significant (p<0.01 in the absence and p<0.05 in the presence of daunomycin, respectively). (C) The ATPase activities of LmrCD in the presence of DARPin_Act2 and E3_5 were determined over a range of ATP concentrations. The data points were fitted to the Hill equation.