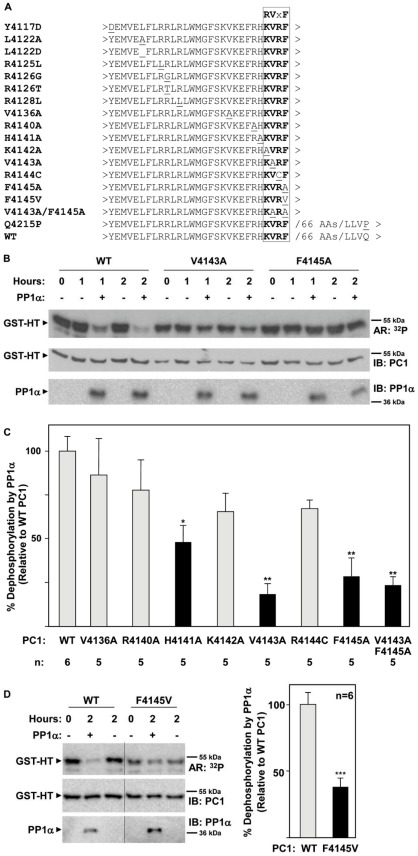
Figure 6. Mutations within the RVxF motif prevent dephosphorylation of PC1 by PP1α.
To determine whether mutations in the RVxF motif affect the ability of PP1α to dephosphorylate PC1, GST-HT193 fusion proteins with point mutations within and around this motif and GST-HA74 were analyzed in an in vitro kinase/phosphatase assay as described in Figure 4. The amount of phosphorylated protein (relative to input material at the start of the reaction) remaining after 2 h in the presence or absence of PP1α was determined by autoradiography and immunoblotting. Following detection of the fusion proteins, membranes were stripped and re-probed for the presence of PP1α. (A) Sequence of wild type (WT), and mutant RVxF constructs of PC1. The primary amino acid sequence spanning the various sites of mutagenesis is shown. The identity of the mutated residue is shown at left and underlined in the primary sequence. The RVxF motif is in bold. (B) Representative autoradiographs and immunoblots showing phosphorylation and total protein levels of GST-HT193 WT, V4143A, and F4145A PC1 fusion proteins. (C) Summary of the effects of PC1 mutations on PP1α-mediated dephosphorylation of PC1. On average, approximately 67% of WT input material was dephosphorylated over the 2 h assay period. Data (mean ± SE) represent the percent dephosphorylation of PC1 constructs by PP1α, relative to dephosphorylation of WT PC1 (set to 100%). n = 6 for WT and n = 5 for mutant constructs. *P<0.05 and **P<0.01, compared to the effect of PP1α on WT PC1, determined by one-way ANOVA and the Dunnett multiple comparison post-test. Representative autoradiographs and immunoblots for GST-HT193 V4136A, R4140A, H4141A, K4142A, R4144C, and V4143A/F4145A are shown in Figure S4A. Representative autoradiographs and immunoblots for additional mutants which lacked obvious defects in PP1α dephosphorylation are shown in Figure S4B. (D) Summary and representative autoradiographs and immunoblots showing phosphorylation and total protein levels of GST-HT193 WT and F4145V PC1 fusion proteins. Data (mean ± SE) represent the percent dephosphorylation of PC1 constructs by PP1α, relative to dephosphorylation of WT PC1 (set to 100%). n = 6 for WT and F4145V. ***P<0.001, compared to the effect of PP1α on WT PC1, determined by unpaired t test.