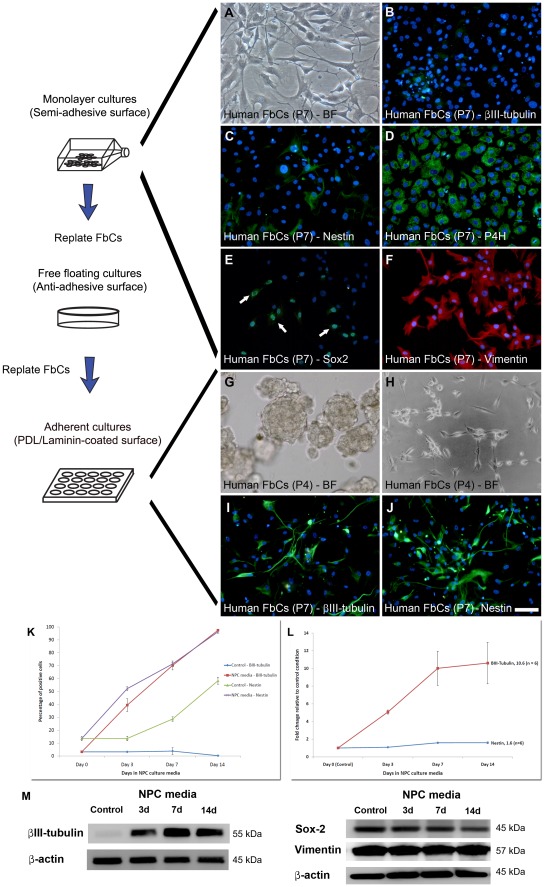
Figure 4. The FbCs isolated from biopsy specimens show NPC-like properties under NPC culture conditions but failed to differentiate into functional neurons.
(A) Bright-field (BF) image showing the distinct morphology of the FbCs. They exhibited a flat and diffuse cytoplasm and were generally phase-dark. These cells showed characteristics of fibroblasts, as they lack the expression of neuronal βIII-tubulin (B), but had high levels of collagen synthesizing enzyme prolyl-4-hydroxylase (P4H; D) and Vimentin (F). However, they also expressed stem cell-like markers such as Nestin (C) and Sox-2 (E). Furthermore, when cultured under neurosphere-forming conditions, FbCs also formed spheres (G) that could be passaged for at least 2 – 3 passages. When FbCs were cultured in NPC culture conditions, their morphology changed to phase-bright polar shaped cells (H) and started expressing high levels of βIII-tubulin (I) and up-regulated their expression of Nestin (J). In agreement with immunostaining results, quantitative image analysis revealed NPC conditions gradually increased the percentage of FbCs expressing Nestin and βIII-tubulin at a detectable level (K). When compared to the control astrocytic culture condition, these differences were significant (P<0.01). Q-RT-PCR data also showed βIII-tubulin mRNA levels increased by over 10 fold by 14 days of exposure to NPC conditions (L; P<0.01). Due to the relatively high basal levels of Nestin in the control cells, the relative increase in Nestin was only 1.5 fold, but this still reached significance (L; P<0.01). Finally, western blots validated many of the above observations, as in control conditions, they showed βIII-tubulin at near un-detectable levels, but increased significantly during the first 3 days in NPC media and continued to increase till day 7 (M). Sox-2 and Vimentin were highly expressed in control and NPC media-induced FbCs (M). These results were consistently observed in all the cases tested (n = 5). Scale: 100 µm.