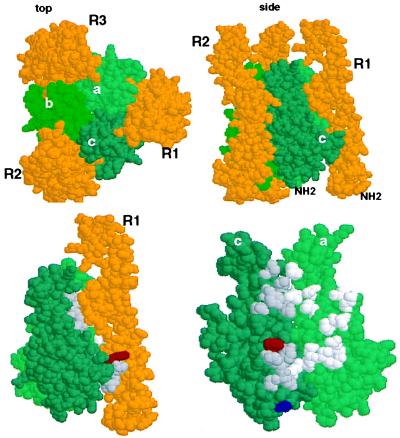
Figure 2.
The E218G allelic substitution lies in the receptor binding region of Fas Ligand. (Upper) Space-filling depiction of LTα TNFR60 ligand–receptor complex from the crystal structure derived by Banner et al. (17) (viewed with RasMol). The three receptor (R) chains (gold) surround the LTα subunits (green) that form the trimeric ligand. The upper left panel (top) is viewed from the perspective of the receptor-expressing cell with the receptor’s N terminus extending away from the reader; in the right panel (side) the N terminus of the elongated receptor protrudes away from the cell surface. (Lower) Location of the T184A and E218G polymorphisms of FasL in the structure of LTα. Residues Phe-110 (red) and Ser-70 (blue) of LTα are equivalent to FasL 218 and 184 as identified by sequence alignment of TNF, LTα, and FasL (Pam250 matrix) and constrained by positions of conserved residues in the D-E and B-C β-strands of LTα. [β-strand nomenclature is that defined by Eck (37)]. Left side shows the ligand-receptor complex (side view) and right side depicts the binding site (with R1 removed) rotated 90° clockwise, exposing the contact residues. Amino acids that contact receptor with a surface area >20 Å2 (17) in the “a” and “c” LTα subunits (“c” subunit, dark gray; “a” subunit, light gray).