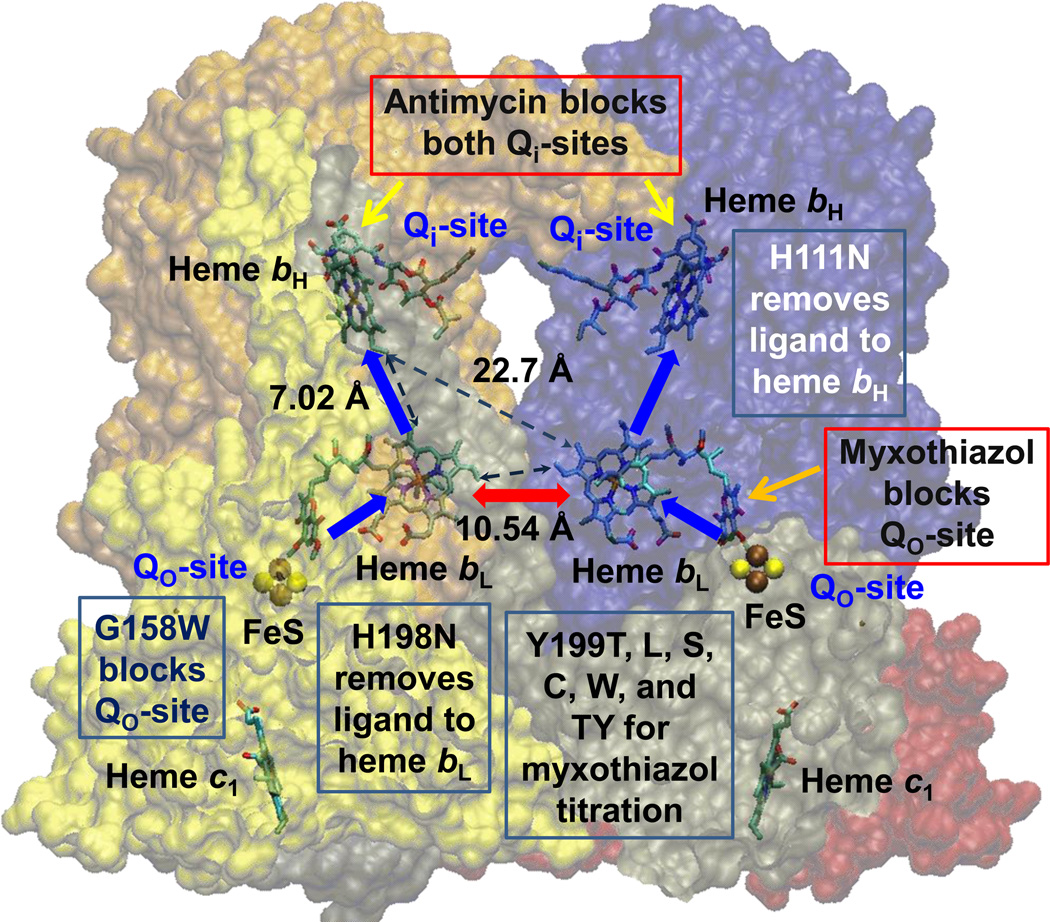
Fig. 1. Scheme to show rationale for the study of inter-monomer electron transfer.
With both Qi-sites blocked by antimycin, electrons cannot exit from heme bH. If one monomer is blocked by myxothiazol at the Qo-site, the unblocked Qo-site would deliver electrons to both monomers if inter-monomer electron transfer could occur rapidly between the bL hemes (red arrow). Blue arrows show monomeric electron transfer. Mutations used to block different partial process were G158W (Qo-site), H111N (heme bH), and H198N (heme bL). See also Table 2 and Fig S1. Structure is from PDB 2QJY [66], in which occupancy by stigmatellin defines the Qo-site, and occupancy by ubiquinone-10 defines the Qi-site.