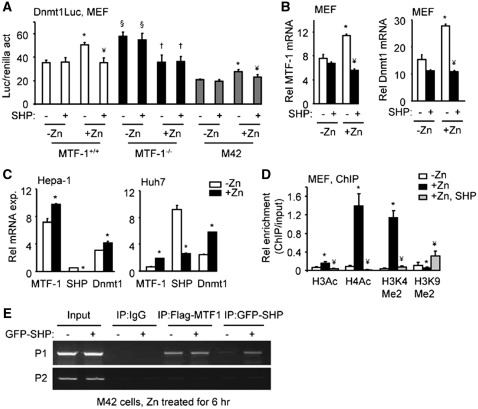
Figure 5.
SHP inhibition of MTF-1-dependent induction of Dnmt1. (A) Transient transfection assays of the Dnmt1 promoter-luciferase (Luc) reporter in MEF (MTF-1+/+, MTF-1−/− and M42) cells without (−Zn) or with 100 µM ZnSO4 treatment for 6 hr (+Zn) and in the absence (−) or presence (+) of SHP over-expression. *P < 0.01, +Zn group versus −Zn group without SHP; ¥P < 0.01, +Zn with SHP versus +Zn without SHP; §P < 0.01, −Zn group in MTF-1−/− versus −Zn group in MTF-1+/+ cells; †P < 0.01, +Zn group versus −Zn group in MTF-1−/− cells. (B) qPCR analysis of MTF-1 and Dnmt1 mRNA in MTF-1+/+ MEF cells without (−Zn) or with 100 µM ZnSO4 treatment for 6 hr (+Zn) and without (−) or with SHP over-expression (+). *P < 0.01, +Zn group versus −Zn group without SHP; ¥P < 0.01, +Zn with SHP versus +Zn without SHP. (C) qPCR analysis of MTF-1, SHP and Dnmt1 mRNA in Hepa-1 and Huh7 cells without (−Zn) or with 100 µM ZnSO4 treatment for 6 hr (+Zn). *P < 0.01, +Zn group versus −Zn group. (A–D) Statistical results represent mean ± SD of triplicate assays. (D) ChIP assays of histone modifications in the Dnmt1 promoter after zinc treatment and SHP over-expression. MEF cells were cultured in medium with (+Zn) or without (−Zn) 100 µM ZnSO4 for 6 hr. ChIP PCR products were amplified from input-positive controls, IgG-negative controls and antibody immunoprecipitates. Histograms show antibody/input ratios of PCR products quantified using qPCR, expressed as Relative (Rel) enrichment. The P1 primers covering the three MREs (see Figure 4D) were used for ChIP assays. Error bars represent SD from three independent measurements. *P < 0.01 versus −Zn group; ¥P < 0.01 versus +Zn group. (E) ChIP assays to determine the effect of SHP expression on the recruitment of MTF-1 to the Dnmt1 promoter. M42 cells were transfected with or without GFP-SHP plasmid for 36 hr and treated with 100 µM ZnSO4 for 6 hr. The chromatin was immunoprecipitated using anti-GFP or anti-Flag antibodies. PCR was used to determine the association of SHP and MTF-1 to the endogenous Dnmt1 promoter by using P1 and P2 primers.