Table 2.
Optimization of reactant, ligand, and reaction conditions for the enantioselective Mukaiyama-Michael addition.[a]
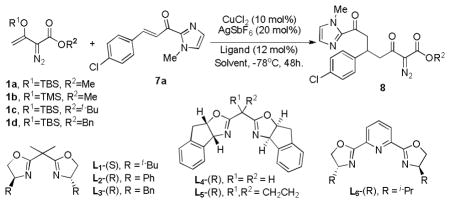 | ||||||
|---|---|---|---|---|---|---|
| entry | 1 | ligand | solvent | additive | yield (%)[b] | ee (%)[c] |
| 1 | 1a | L1 | DCM | - | 43 | 54 |
| 2 | 1b | L1 | DCM | - | 64 | 12 |
| 3 | 1c | L1 | DCM | - | 33 | 50 |
| 4 | 1d | L1 | DCM | - | 42 | 93 |
| 5 | 1d | L2 | DCM | - | 30 | 65 |
| 6 | 1d | L3 | DCM | - | 33 | 76 |
| 7 | 1d | L4 | DCM | - | 40 | 87 |
| 8 | 1d | L5 | DCM | - | 34 | 88 |
| 9 | 1d | L6 | DCM | - | <5 | ND |
| 10 | 1d | L1 | DCM | HFIP (1.0 eq) | 67 | 83 |
| 11 | 1d | L1 | DCM | 4Å (0.1g) | 44 | 94 |
| 12 | 1d | L1 | THF | 4Å (0.1g) | <5 | ND |
| 13 | 1d | L1 | Toluene | 4Å (0.1g) | 35 | 90 |
| 14[d] | 1d | L1 | DCM | HFIP (1.0 eq) | 78 | 93 |
| 15[e] | 1d | L1 | DCM | HFIP & 4Å | 81 | 94 |
Reactions were performed as described in Table 1. The chiral catalyst was prepared according to ref 12.
Isolated yield of 8 after chromatography.
Determined by chiral HPLC (See supporting information).
30 mol% Catalyst was used.
Catalyst was prepared in a glove box, and the reaction was run for three days.
