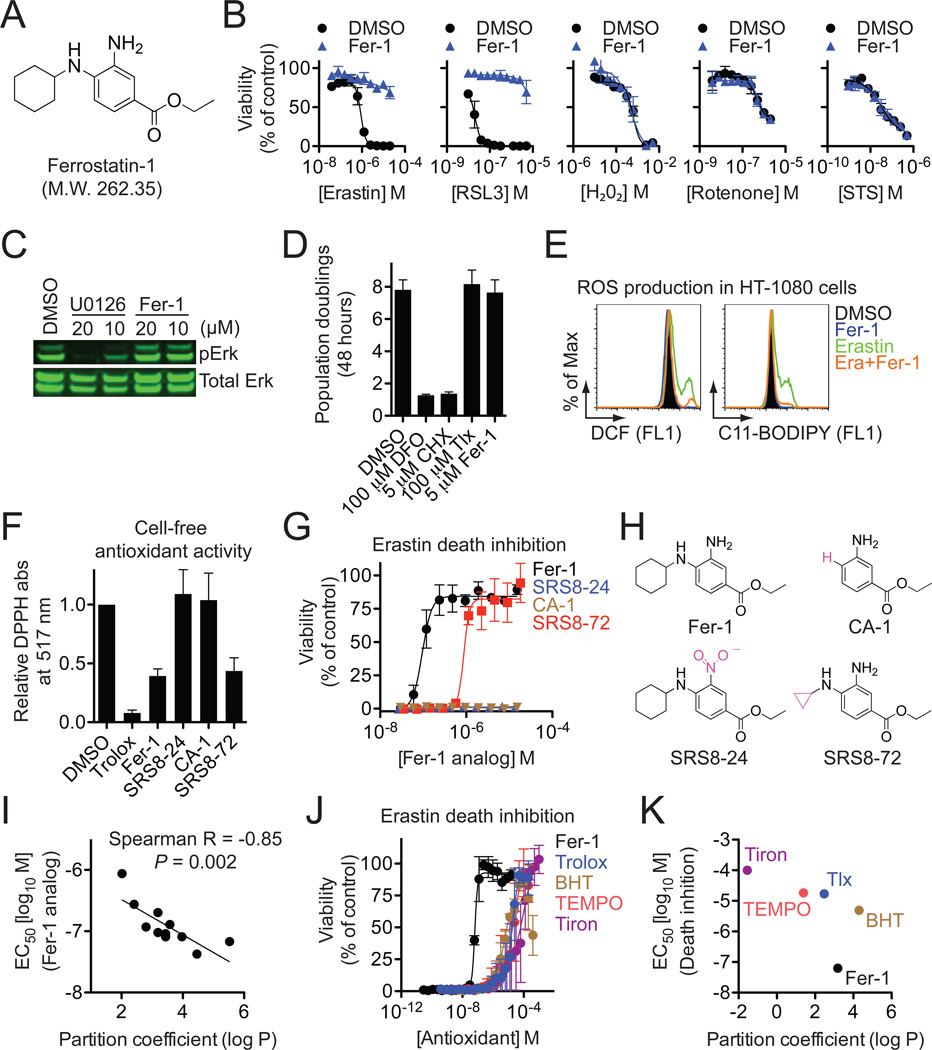
Figure 4. Identification and characterization of Ferrostatin-1.
(A) Structure of ferrostatin-1 (Fer-1). (B) Effect of resynthesized Fer-1 (0.5 µM) on the lethality of various compounds in HT-1080 cells. (C) Effect of Fer-1 and U0126 on ERK phosphorylation in HT-1080 cells. (D) Effect of DFO, CHX, trolox (Tlx) and Fer-1 on HT-1080 cell proliferation over 48 hours as assessed by Vi-Cell. (E) Effect of Fer-1 (0.5 µM) on erastin (10 µM)-induced ROS production in HT-1080 cells (4 hr treatment). (F) Cell-free antioxidant potential monitored by changes is the absorbance at 517 nm of the stable radical DPPH. (G) Dose-response relationship for inhibition of erastin (10 µM, 24 hrs)-induced death in HT-1080 cells by Fer-1 and analogs. (H) Correlation between predicted partition coefficient (log P) and the ability of various Fer-1 analogs to prevent erastin-induced death. (I) Dose-response relationship for inhibition of erastin (10 µM, 24 hrs)-induced death by various antioxidants. (J) Plot of predicted partition coefficient (log P) and ability of various antioxidants to prevent erastin-induced death. Data in (B), (D), (F), (G) and (J) represents mean+/−SD from one of three representative experiments.