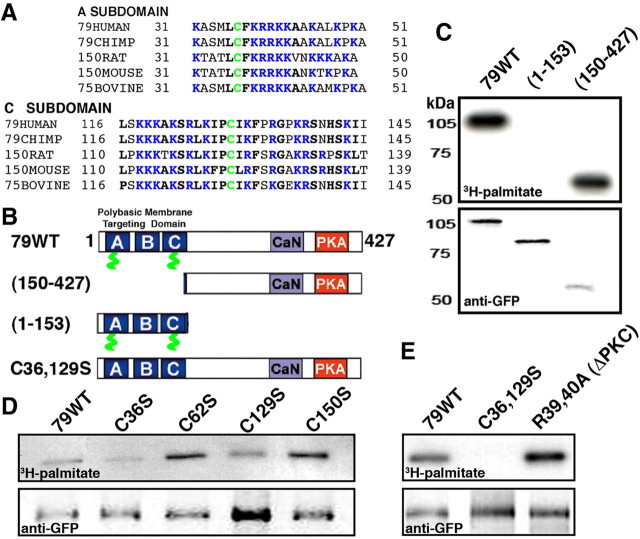
Figure 1.
AKAP79 is palmitoylated on two conserved Cys residues in the N-terminal polybasic membrane targeting domain. A, Sequence alignments of the AKAP79/150 A and C basic targeting subdomains from multiple species showing the locations of C36 and C129 in green. Basic residues are in blue and other highly conserved residues are in black, bold. B, Diagram of selected AKAP79 mutants used for mapping palmitoylation sites. The locations of the A–C basic targeting subdomains (blue), C36 and C129 (green), CaN anchoring domain (purple), and PKA anchoring (red) are indicated. C, COS7 cells transfected with GFP-tagged AKAP79 constructs and labeled with 3H-palmitate detected by fluorography. Palmitoylation (top panel) of the N-terminal 1–153 targeting domain fragment is similar to full-length AKAP79 with no detectable palmitoylation observed for 154–427. Levels of AKAP79 proteins detected by anti-GFP immunoblotting (bottom panel). D, Palmitoylation (top panel) is reduced by C36S and C129S (but not C62S or C150S) mutations within the AKAP79 N-terminal targeting domain. Levels of AKAP79 proteins detected by anti-GFP immunoblotting (bottom panel). E, Palmitoylation (top panel) is prevented by the C36,129S double point mutation but is not impacted by the R39,40A mutation that inhibits nearby PKC binding to AKAP79. Levels of AKAP79 proteins detected with anti-GFP immunoblotting are shown (bottom panel).