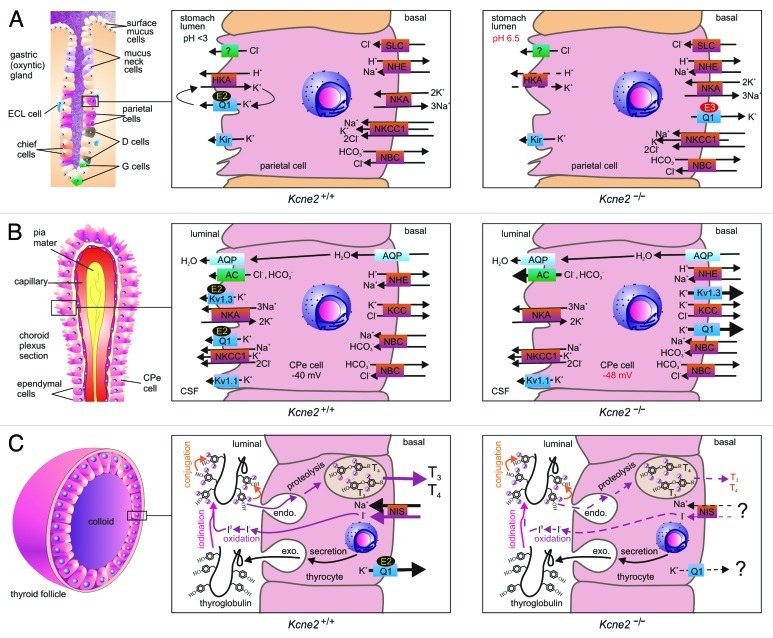
Figure 2. KCNE2 in epithelial cells. (A) KCNE2 in the gastric epithelium. Left, organization of an oxyntic gland in the stomach, with the parietal cell highlighted. Center, the gastric H+/K+-ATPase requires a luminal K+ recycling pathway for gastric acidification, formed by KCNQ1 (Q1) and KCNE2 (E2). Right, in Kcne2−/− mice, KCNQ1 is incorrectly targeted to the parietal cell basolateral membrane by upregulated KCNE3 (E3), and the mice are achlorydric because the gastric H+/K+-ATPase lacks a suitable K+ recycling pathway. (B) KCNE2 in the choroid plexus epithelium. Left, organization of the choroid plexus epithelium (CPe). Center, KCNE2 forms apical K+ channel complexes with KCNQ1 and Kv1.3, which probably contribute to setting membrane potential and regulating anion secretion into the CSF. Right, in Kcne2−/− mice, KCNQ1 and Kv1.3 are incorrectly targeted to the CPe cell basolateral membrane where they pass abnormally large outward K+ currents. Anion secretion appears to be increased, probably because the larger outward K+ current hyperpolarizes the CPe cell Em. (C) KCNE2 in the thyroid epithelium. Left, organization of a thyroid follicle, skirted by thyroid epithelial cells (thryocytes). Center, biosynthesis of thyroid hormones T3 and T4 requires I- to pass from the blood into the central colloid, where it is oxidized and organified by incorporation into thyroglobulin (iodination and conjugation). The product is endocytosed back into the thyrocyte, converted to T3 and T4 by proteolysis, then transported into the blood. KCNQ1-KCNE2 channels form on the basolateral membrane, as does the sodium iodide symporter (NIS). Right, in Kcne2−/− mice (and in Kcnq1−/− mice), thyroid hormone biosynthesis is disrupted by an incompletely understood mechanism characterized by impaired thyroid I- accumulation.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.