Abstract
Optimal investment in offspring is important in maximizing lifetime reproductive success. Yet, very little is known how animals gather and integrate information about environmental factors to fine tune investment. Observing the decisions and success of other individuals, particularly when those individuals initiate breeding earlier, may provide a way for animals to quickly arrive at better breeding investment decisions. Here we show, with a field experiment using artificial nests appearing similar to resident tit nests with completed clutches, that a migratory bird can use the observed high and low clutch size of a resident competing bird species to increase and decrease clutch size and egg mass, accordingly. Our results demonstrate that songbirds can discriminate between high and low quantity of heterospecific eggs, and that social information can have long-term physiological consequences affecting reproductive strategies. Such behaviour may help animals to better adapt to changing environments and lead to convergent traits with competitors.
Keywords: social information use, species interactions, offspring investment, clutch size, interspecific competition
1. Introduction
Optimal investment in current offspring is crucial for maximizing lifetime reproductive success [1]. Making optimal decisions, however, is difficult because of spatial and temporal variation in factors affecting the fecundity and survival of both breeding adults and their offspring. Acquiring personal information from all relevant factors, such as amount and quality of food, intra- and interspecific competition, parasitism and predation, would provide the most accurate estimates but entails costs in terms of energy, survival and time [2]. Therefore, one could expect that animals should have more integrated, quicker assessing mechanisms. Recent studies have indeed revealed that the decisions of both conspecifics [3] and heterospecifics [4] are used in guiding own behaviour.
Offspring investment of conspecifics could provide relevant information but it is rarely available owing to synchronous breeding and territoriality. The investment of heterospecifics with similar resource needs—potential competitors—that are ahead in the reproduction cycle is available, and can potentially provide an integrated measure of habitat quality. This is because first, females often adjust offspring investment relative to environmental factors, such as food and habitat quality [5–9], but see Svensson & Nilsson [10], and in particular offspring mortality [11–13]. Second, offspring number is often positively associated with the quality of the environment because higher quality and/or older individuals usually have a higher number of offspring [5,10] and they also often occupy higher quality territories [14].
We examined with a field experiment whether pied flycatchers (Ficedula hypoleuca) adjust offspring investment relative to the observed clutch size of a potential competitor, the great tit (Parus major). The tit clutch size information is readily available because most tits are far in egg laying or are incubating when flycatchers arrive on their breeding grounds. Flycatchers also frequently visit tit nests upon arrival (O. Loukola, J. T. Forsman & J.-T. Seppänen 2011, unpublished data), despite the risk of injury or death from aggressive tits [15]. The clutch size of tits is often associated with habitat quality and food [5,8,16], but see Svensson & Nilsson [10] and predation risk [11]. Pied flycatchers can also respond to observed tit clutch sizes [17,18] when choosing breeding site features.
We used simulated great tit nests with two clutch size treatments corresponding to the highest and lowest naturally occurring clutch sizes. We predicted that if flycatchers use the clutch size of tits as a cue in their own investment decisions, birds breeding at sites with the high simulated clutch size treatment should invest more in offspring (higher clutch size, heavier eggs and clutch) than flycatchers in the low clutch size treatment.
2. Material and methods
(a). Experimental design
The experiment was conducted in Oulu, northern Finland, in two years (2009 and 2010) during the settlement period of flycatchers. The experiment included two treatments: simulated great tit nests with plastic eggs with high (13 eggs) and low (four eggs) clutch size. Each experimental site was isolated from other breeding tits and included only one nest box with an artificial tit nest and at least one empty nest box for a flycatcher pair. Experimental sites were far apart (greater than 400 m) to ensure independence between sampling units. Details of the design not related to the hypothesis addressed here differed between years owing to other concurrent studies, but these had no effect on the measured response variables (electronic supplementary material, appendix A).
All nest boxes at a given site were alike and attached to similar trees. The treatment of the set-up and the nest box to include the simulated tit nest were randomly assigned. The location of the sites was changed between years. Simulated tit nests were made of moss and animal hair, which are natural materials of tit nests. Plastic eggs (Canary Nest Egg, Solway Feeders, UK) were painted pale with brown speckles to resemble tit eggs. In contrast, flycatcher nests are composed of grass and bark, and their eggs are pale blue, so flycatchers were unlikely to confuse a simulated nest with a conspecific nest. Eggs were left exposed in the nest cup to mimic initiated incubation because usually tits cover their eggs during, but not after egg laying.
This design controls for or randomizes many factors affecting offspring investment. First, using simulated nests at spatially isolated sites removes the effects of tit density or behaviour (e.g. aggressiveness and competitive ability) that may affect flycatcher decisions [19,20]. Second, randomly assigned treatments control the potential effect of different habitat quality on clutch size. Third, using simulated nests with plastic eggs ensures that the observed stimulus is similar for all flycatchers.
The following data were gathered on flycatcher breeding: initiation of egg laying, clutch size, mass of the eggs (measured prior to the incubation with a digital scale; Jscale JS-100xV, accuracy 0.01 g) and the age of females.
(b). Analyses
Treatment effects on the occupancy of the sites and consistency between years were examined by conducting a logistic regression analysis in which the occupancy of the site (occupied/unoccupied) was explained by the treatment, study year and their interaction.
We used generalized linear modelling to analyse the effects of treatments on clutch size, average mass of the egg and the total mass of the eggs in a clutch (clutch mass). Depending on the dependent variable, we also included female age, year, initiation day of egg laying and clutch size into the initial model, and all relevant interactions. We used the Akaike information criteria [21] to reach the most parsimonious model.
3. Results
In 2009, 53 out of 90 available sites became occupied (64.4%), whereas in 2010, 38 out of 63 sites became occupied (60.3%). Treatment did not affect the odds of occupancy (logistic regression: likelihood-ratio of the model = 1.847, d.f. = 3, p = 0.605) and treatment effects on occupancy were similar in both years (treatment by year interaction: likelihood-ratio = 0.312, d.f. = 3, p = 0.958). Of the settled females, 80 females in total (43 in 2009 and 37 in 2010) succeeded to proceed through laying to incubation, and these were used in further analyses.
The simulated great tit clutch size affected flycatcher investment decisions as predicted. For the clutch size, female age had the strongest effect (Wald χ2 = 16.12, d.f. = 1, p < 0.000), but the treatment was also included into the final model (Akaike index difference, ΔAIC, to the next best model 0.45). Females breeding at high simulated tit clutch size set-ups laid on average 0.42 eggs (6.9%) more than those at low clutch size treatment (figure 1a; Wald χ2 = 4.12, d.f. = 1, p = 0.042).
Figure 1.
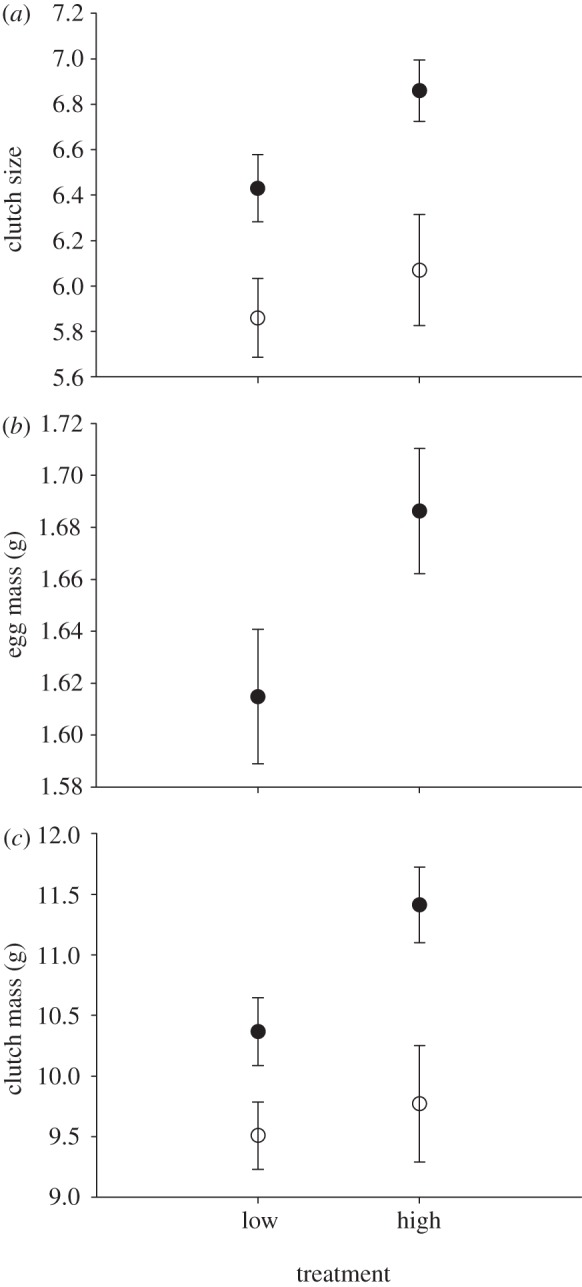
Flycatcher investment decisions in the two treatments. Females laid (a) more (b) heavier eggs (in 2009) and (c) clutches at simulated high (13 eggs) tit clutch size treatment than in the low (four eggs) clutch size treatment. Black and white circles refer to adult and young females, respectively. Female age did not affect average egg mass and therefore the data was pooled across age classes. Error bars indicate standard error of the mean.
The initial full model for average egg mass included a statistically significant interaction term between the treatment and the year (Wald χ2 = 5.15, d.f. = 1, p = 0.023), suggesting that the effect of treatment was different between years. Therefore, years were analysed separately. In 2009, the best model included only the treatment (ΔAIC = 0.17) and females in the high simulated clutch size treatment laid on average 0.072 g (4.5%) heavier eggs than in the low clutch size treatment (Wald chi-square = 4.21, d.f. = 1, p = 0.040; figure 1b). In 2010, the best model also included only the treatment but its effect was not statistically significant (Wald χ2 = 2.16, d.f. = 1, p = 0.141).
Clutch mass was affected by female age (Wald χ2 = 11.33, d.f. = 1, p = 0.001), date of initiation of egg laying and the treatment (ΔAIC = 1.45). Clutch mass was reduced with the progress of the breeding season (initiation of egg laying: Wald χ2 = 3.60, d.f. = 1, p = 0.058). Females breeding at high simulated clutch size treatment had 9.3% (0.92 g) higher clutch mass than females in the low clutch size treatment (Wald χ2 = 3.64, d.f. = 1, p = 0.056; figure 1c).
4. Discussion
Our main findings are that pied flycatchers can perceive and respond to observed heterospecific clutch sizes and adjust their own offspring investment decisions accordingly.
The observed response is line with our earlier findings [17,18] and implies that flycatchers are capable of quantitative discrimination of heterospecific clutch size and further emphasize the role of clutch size as a valuable source of information. The experiment cannot provide a definitive answer about the mechanism, but the somewhat stronger response of adult females to the treatments compared with females breeding for the fist time suggests that individual experience may be involved. Adult females have most likely visited more tit nests and thereby might have empirically adjusted reference to the quantity of tit eggs. They also have personal experience about their own clutches (median six eggs) and incubation, which may give them reference against which to compare the quantity of tit eggs.
Optimal investment in reproduction given the abundance of food has been studied intensively [1], yet the evidence for animals' capability for adaptive adjustments is mixed [7]. Even effects of mortality factors on investment are not always straightforward. For example, even though nest predation is the major cause of nesting failures in birds [22], Fontaine & Martin [12] found no effect of experimentally reduced nest predator abundances on the clutch sizes of 12 arboreal bird species; the expected adjustment was instead found in egg and clutch mass. In this context, the observed effect of simulated tit clutch size on flycatcher clutch size and egg mass in 2009 (difference of 6.9% and 4.5% between treatments, respectively) is biologically significant.
We suggest that ability to respond to observed heterospecific clutch size is adaptive as it aids females to estimate territory quality and adjust own offspring investment accordingly, which may have large fitness benefits [23]. Our earlier studies demonstrated that flycatchers also respond to tit clutch sizes in selecting nest site features [17,18]. In concert, these results suggest that the observed relative size of a heterospecific clutch can be an important source of information affecting not only the immediate behaviour, but also long term physiological mechanisms determining the clutch size and egg mass. The ability to observe breeding investment and success of the earlier breeding species are common in nature and could affect many reproductive decisions. If the offspring investment of the species providing information honestly reflects environmental conditions, then interspecific information may help information users to better adapt to changing environments.
Acknowledgements
We acknowledge the financial support provided by the Academy of Finland (project nos. 122665 and 125720). We thank Mikko Mönkkönen for fruitful comments and Sami Kivelä for statistical advice.
References
- 1.Roff D. A. 1992. The evolution on life histories: theory and analysis. London, UK: Chapman and Hall [Google Scholar]
- 2.Stamps J. A., Krishnan V. V., Reid M. L. 2005. Search costs and habitat selection by dispersers. Ecology 86, 510–518 10.1890/04-0516 (doi:10.1890/04-0516) [DOI] [Google Scholar]
- 3.Danchin E., Giraldeau L. A., Valone T. J., Wagner R. H. 2004. Public information: from nosy neighbors to cultural evolution. Science 305, 487–491 10.1126/science.1098254 (doi:10.1126/science.1098254) [DOI] [PubMed] [Google Scholar]
- 4.Seppänen J.-T., Forsman J. T., Mönkkönen M., Thomson R. L. 2007. Social information use is a process across time, space and ecology, reaching heterospecifics. Ecology 88, 1622–1633 10.1890/06-1757.1 (doi:10.1890/06-1757.1) [DOI] [PubMed] [Google Scholar]
- 5.Perrins C. M. 1965. Population fluctuation and clutch size in the great tit, Parus major L. J. Anim. Ecol. 34, 601–647 10.2307/2453 (doi:10.2307/2453) [DOI] [Google Scholar]
- 6.Högstedt G. 1980. Evolution of clutch size in birds: adaptive variation in relation to territory quality. Science 210, 1148–1150 10.1126/science.210.4474.1148 (doi:10.1126/science.210.4474.1148) [DOI] [PubMed] [Google Scholar]
- 7.Martin T. E. 1987. Food as a limit on breeding birds: a life-history perspective. Annu. Rev. Ecol. Syst. 18, 453–487 10.1146/annurev.es.18.110187.002321 (doi:10.1146/annurev.es.18.110187.002321) [DOI] [Google Scholar]
- 8.Dhondt A. A., Kempenaers B., Adriaensen F. 1992. Density-dependent clutch size caused by habitat heterogeneity. J. Anim. Ecol. 61, 643–648 10.2307/5619 (doi:10.2307/5619) [DOI] [Google Scholar]
- 9.Risch T. S., Michener G. R., Dobson F. S. 2007. Variation in litter size: a test of hypotheses in Richardson's ground squirrel. Ecology 306, 306–314 10.1890/06-0249 (doi:10.1890/06-0249) [DOI] [PubMed] [Google Scholar]
- 10.Svensson E., Nilsson J.-Å. 1995. Food supply, territory quality, and reproductive timing in the blue tit (Parus caeruleus). Ecology 76, 1804–1812 10.2307/1940712 (doi:10.2307/1940712) [DOI] [Google Scholar]
- 11.Julliard R., McCleery R. H., Clobert J., Perrins C. M. 1997. Phenotypic adjustment of clutch size due to nest predation in the great tit. Ecology 78, 394–404 10.1890/0012-9658(1997)078[0394:PAOCSD]2.0.CO;2 (doi:10.1890/0012-9658(1997)078[0394:PAOCSD]2.0.CO;2) [DOI] [Google Scholar]
- 12.Fontaine J. J., Martin T. E. 2006. Parent birds assess nest predation risk and adjust their reproductive strategies. Ecol. Lett. 9, 428–434 10.1111/j.1461-0248.2006.00892.x (doi:10.1111/j.1461-0248.2006.00892.x) [DOI] [PubMed] [Google Scholar]
- 13.Mönkkönen M., Forsman J. T., Kananoja T., Ylönen H. 2009. Indirect cues of nest predation risk and avian reproductive decisions. Biol. Lett. 5, 176–178 10.1098/rsbl.2008.0631 (doi:10.1098/rsbl.2008.0631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alatalo R. V., Lundberg A., Glynn C. 1986. Female pied flycatchers choose territory quality and not male characteristics. Nature 323, 152–153 10.1038/323152a0 (doi:10.1038/323152a0) [DOI] [Google Scholar]
- 15.Merilä J., Wiggins D. A. 1995. Interspecific competition for nest holes causes adult mortality in the collared flycatcher. Condor 97, 445–450 10.2307/1369030 (doi:10.2307/1369030) [DOI] [Google Scholar]
- 16.Nilsson J.-Å. 1991. Clutch size determination in the marsh tit (Parus palustris). Ecology 72, 1757–1762 10.2307/1940974 (doi:10.2307/1940974) [DOI] [Google Scholar]
- 17.Seppänen J.-T., Forsman J. T., Mönkkönen M., Krams I., Salmi T. 2011. New behavioural trait adopted or rejected by observing heterospecific tutor fitness. Proc. R. Soc. B 278, 1736–1741 10.1098/rspb.2010.1610 (doi:10.1098/rspb.2010.1610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forsman J. T., Seppänen T. 2011. Learning what (not) to do: testing rejection and copying of heterospecific behavioural trait. Anim. Behav. 81, 879–883 10.1016/j.anbehav.2011.01.029 (doi:10.1016/j.anbehav.2011.01.029) [DOI] [Google Scholar]
- 19.Van Duyse E., Pinxten R., Eens M. 2002. Effects of testosterone on song, aggression and nestling feeding behavior in male great tits, Parus major. Horm. Behav. 41, 178–186 10.1006/hbeh.2001.1747 (doi:10.1006/hbeh.2001.1747) [DOI] [PubMed] [Google Scholar]
- 20.Forsman J. T., Hjernquist M. B., Taipale J., Gustafsson L. 2008. Competitor density cues for habitat quality facilitating habitat selection and investment decisions. Behav. Ecol. 19, 539–545 10.1093/beheco/arn005 (doi:10.1093/beheco/arn005) [DOI] [Google Scholar]
- 21.Burnham K. P., Anderson D. R. 2002. Model selection and multi-model inference: a practical information theoretic approach, 2nd edn. New York, NY: Springer [Google Scholar]
- 22.Martin T. E. 1995. Avian life history evolution in relation to nest sites, nest predation, and food. Ecol. Monogr. 65, 101–127 10.2307/2937160 (doi:10.2307/2937160) [DOI] [Google Scholar]
- 23.Williams T. D. 1994. Intraspecific variation in egg size and egg composition in birds; effects on offspring fitness. Biol. Rev. Camb. Philos. Soc. 68, 35–59 [DOI] [PubMed] [Google Scholar]


