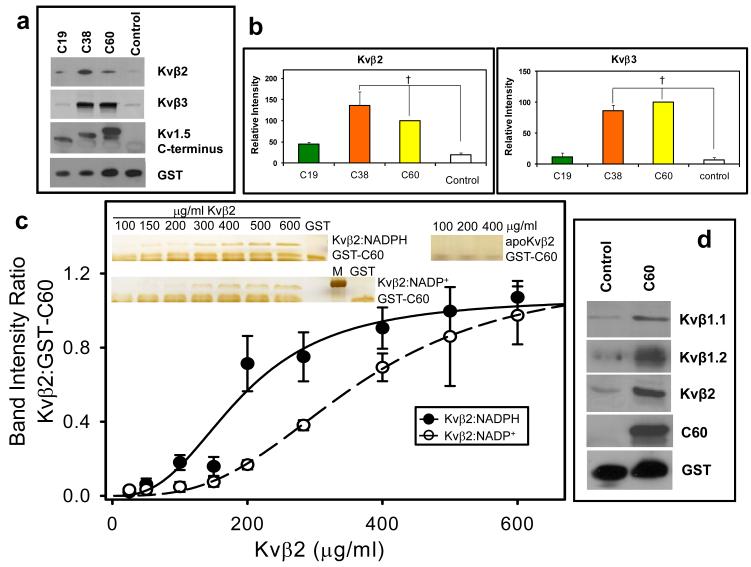
Fig. 8. Binding of the C-terminal domain of Kv to Kvβ.
(a) Western blots of Kvβ2 (upper panel) and Kvβ3 (middle panel) pulled down by the GST-Kv1.5 C-terminus fusion peptides. Fusion proteins containing 60, 38, or 19 terminal amino acid peptides from Kvα1.5 C-terminus attached to GST or GST with unrelated peptide (Control; 30μ g each) were incubated with lysate of Kvβ2 or Kvβ3 -expressing E.coli (350 μ g total protein). Protein complexes were pulled down using GST·Bind beads, washed and eluted with 10mM glutathione. The eluate was separated by SDS-PAGE and probed with anti-pan-Kvβ antibody, an antibody directed against the C-terminus of Kv1.5 (bait) or GST; (b) Densitometric analysis of the bands in panel a. The density of the Kvβ band precipitated with GST-C60 was assigned a 100% value. †, P< 0.05 versus Control peptide (n=3-5). (c) Determination of the binding affinity of Kvβ:nucleotide complexes to the C-terminal peptide of Kv1.5. A fixed concentration of GST-C60 (30 μ g/ml) was mixed with variable concentrations of Kvβ2 in a binary complex with NADPH (Kvβ2:NADPH), or NADP+ (Kvβ2:NADP+), or none (apo-Kvβ2) and GST pull down assay was performed. Eluted protein was separated by SDS-PAGE and the gels were silver stained. Band intensities normalized to GST-C60 input were plotted as a function of Kvβ2 concentration. The experiment was repeated 6 times; data points on the graph represent average and standard error, and the lines represent the best fit to experimental data using the Hill equation. Apo:Kvβ2 did not bind GST-C60 and no measurable intensities were found by silver stain. Inset: silver stained gels showing Kvβ2 in complex with NADPH, NADP+ or without nucleotide, pulled down with GST-C60; M, marker, 37 kDa band is shown. (d) Western blots of GST beads incubated with brain lysates. The GST-C60 construct or scrambled construct was used to pull down proteins from mouse brain extract. The eluate was separated on SDS PAGE and protein bands were visualized with antibodies against the indicated Kvβ isoform, Kv1.5 C-terminus, or GST.