Abstract
Glioblastoma multiforme (GBM) is the most common malignant brain tumor and is characterized by high invasiveness, poor prognosis, and limited therapeutic options. Biochemical and morphological experiments have shown the presence of caveolae in glioblastoma cells. Caveolae are flask-shaped plasma membrane subdomains that play trafficking, mechanosensing, and signaling roles. Caveolin-1 is a membrane protein that participates in the formation of caveolae and binds a multitude of signaling proteins, compartmentalizing them in caveolae and often directly regulating their activity via binding to its scaffolding domain. Caveolin-1 has been proposed to behave either as a tumor suppressor or as an ongogene depending on the tumor type and progress. This review discusses the existing information on the expression and function of caveolin-1 and caveolae in GBM and the role of this organelle and its defining protein on cellular signaling, growth, and invasiveness of GBM. We further analyze the available data suggesting caveolin-1 could be a target in GBM therapy.
Keywords: caveolae, caveolin-1, EGF receptor, glioblastoma, uPA
Caveolin-1 and Caveolae
Caveolae are plasma membrane subdomains of distinct lipid and protein compositions present in many mammalian cells. These flask-shaped organelles play multiple roles in cell physiology, including serving as signaling platforms for numerous pathways, as clathrin-independent routes of endocytosis, and as mechanical stress sensors.1 The functions of caveolae require proteins of the caveolin family and, most importantly, caveolin-1, a membrane integral protein key to caveola structure. Caveolins oligomerize and, in concert with cytoplasmic proteins of the cavin family, allow caveolae to form.2,3 Caveolin-1 exhibits an unusual conformation with a short membrane-inserted domain and its N- and C- termini both facing the cytosol. Caveolin-1 C-terminus is triply palmitoylated,4 and the N-terminus encompasses a putative cholesterol-binding domain,5 a scaffolding domain,6 and functionally important serine7 and tyrosine8 phosphorylation sites.
In addition to participating in caveola formation, caveolin-1 is known to directly interact via its scaffolding domain with multiple signaling proteins and to regulate their activity. These proteins include important regulators of cell transformation and growth.9 Furthermore, caveolin-1 is best known as a membrane protein, but noncaveolar, soluble, and secreted forms of caveolin-1 have also been described and seem to have biological functions (reviewed in Parat1). Both caveolar and noncaveolar caveolin-1 are involved in modulating cancer cell growth and metastasis. This is reviewed elsewhere9–12 and will only be briefly mentioned to keep our review focused on glioblastoma multiforme (GBM).
Caveolin-1 has been proposed to behave as a tumor suppressor or an ongogene, depending on the tumor type. Initially, caveolin-1 was regarded as a tumor suppressor. Its expression increased with differentiation, and its loss was associated with de-repression of growth-promoting signaling. The gene encoding caveolin-1 was mapped to the human chromosome 7q31.1, where a known fragile site frequently deleted in human cancers is also located.13 Mutation or loss of caveolin-1 expression because of heterozygosity or promoter hypermethylation were described in multiple cancers, such as breast, ovarian, small cell lung, small cell bladder, or colorectal carcinomas (reviewed in van Golen9). In contrast, overexpression of caveolin-1 was reported in a number of malignancies, including prostate cancer. In prostate cancer, caveolin-1 expression and secretion are increased and caveolin-1 contributes to tumor angiogenesis, growth, and metastasis.14–16 In addition to being tumor type-specific, caveolin-1 expression is now accepted to be tumor stage-specific, with decreased expression favoring proliferation and survival at early stages, followed by upregulated expression accompanying invasiveness, metastasis, and multidrug resistance at later stages.12 A distinct role for caveolin-1 in tumor cells versus caveolin-1 expressed by stromal cells is emerging, bringing increasing complexity to the picture.17,18 Lastly, recent evidence suggests that there might be a different role and a different significance for caveolin-1 in cancer cells, depending on its subcellular localization, with noncaveolar caveolin-1 increasing tumor aggressiveness.19,20
In addition to caveolins, caveola formation and functions are now known to involve a family of cytoplasmic proteins named cavins. Cavin-1, or polymerase I and transcript release factor (PTRF), is the only cavin indispensable for caveola formation.2,21 It also plays roles in transcription termination via interaction with RNA polymerase I22,23 and in regulation of type I collagen gene expression by interacting with a DNA-binding transcription factor.24 Of the other members of the cavin family, the serum-deprivation response (SDPR/cavin-2) participates in caveolar membrane curvature,25 the SDR-related gene product that binds to C kinase (SRBC/cavin-3) regulates caveolar budding,26 and the muscle restricted coiled-coil protein (MURC/cavin-4) is muscle specific.21 Dysregulated expression of proteins from the cavin family in cancer is documented.27,28 The expression of PTRF usually mirrors that of caveolin-1 in normal and tumor tissue.2,21,27 When not expressed, however, the absence of PTRF results in the expression of noncaveolar caveolin-1,2 which may have functional consequences.19,29 PTRF was identified as a gene increasing chemoresistance of colorectal cancer cells.30 Of interest, PTRF was detected in a phosphoproteomic study as a substrate in which tyrosine phosphorylation increases 5-fold in cells expressing constitutively active forms of the EGF receptor.31 The significance of PTRF tyrosine phosphorylation is unknown at present.
Caveolae and caveolin-1 regulate signaling molecules by various mechanisms.1 Compartmentalization in caveolae promotes optimal signal transduction via proximity of signaling partners, but several caveolin-1–binding proteins (including receptor tyrosine kinases, heterotrimeric G-proteins, Src family tyrosine kinases, and H-ras) are tonically inhibited by caveolin-1. Some signaling molecules can further be endocytosed via caveolae. Lack of caveolin-1 can result in de-repression of a normally inactive pathway that is maintained by the binding of caveolin-1 to a signaling molecule. For example, the epidermal growth factor receptor (EGFR) is proposed to accumulate in caveolae, where it is inhibited by interaction with the caveolin-1 scaffolding domain.32,33 In this model, in caveolae, the EGFR is in proximity with downstream signaling molecules, such as Ras. After stimulation, the EGFR moves away from caveolae domains, Raf-1 is recruited to Ras, and activation of the Ras/Raf-1/MAPK pathway is initiated within caveolae.32 This pathway is further controlled downstream by direct inhibition of the kinase activity of MEK-1 and ERK-2 by caveolin-1.34 Of note, caveolar localization of EGFR is not unanimously accepted.35 Caveolin-1 deficiency also results in a lack of caveolae that can cause a loss of proximity between signaling molecules that need compartmentalization for efficient signal transduction.36 Furthermore, the absence of caveolae can alter the internalization of receptors or effectors whose subcellular localization is essential for activity.
Caveolin-1 is a substrate for kinases of the Abl and Src families. Tyrosine 14 phosphorylation of caveolin-1 occurs after integrin ligation, growth factor stimulation (including EGF stimulation), and oxidative stress37 and has been linked to cell migration via a series of experiments, showing that caveolin-1 polarization in transmigrating cells requires Tyr14,38,39 and that Tyr14 phosphorylated caveolin-1 confers binding to SH2-containing proteins regulating cell motility40,41 and plays a structural and signaling role at focal adhesions.42,43 Caveolin-1 Tyr14 further mediates mechanotransduction.44 In addition, caveolin-1 phosphorylation is associated with endocytosis, including integrin-mediated caveolae internalization, which when deregulated, can allow anchorage-independent growth.45
Expression and Functions of Caveolin-1 in Astrocytes
Much less is known about caveolin-1 expression and functions in the different cell types of the brain, compared with other cell types, in part because the brain expresses much less caveolin-1 than do other tissues. However, caveolin-1 expression has been documented in neurons and glia. The abundance of caveolin-1 mRNA varies in different parts of the brain.46
Caveolae have been observed in early studies of optic nerve astrocytes using thin section and freeze fracture electron microscopy and were abundant in areas of the plasma membrane facing other astrocytes (up to 17 caveolae per micrometer2) or opposed to myelin sheaths.47 Caveolae were also found in host astrocytes invading neural transplants48 or in cultured rat primary astrocytes.49 In a comprehensive study of caveolin-1 expression in astrocytes, caveolin-1 alpha (full length) isoform was detected by Western blot analysis in low-density, detergent-insoluble microdomains from type 1 astrocytes. Immunofluorescence microscopy of primary cells revealed a punctuate pattern resembling that of other caveolin-1-expressing cells. Caveolin-1 messenger RNA was detected in primary astrocytes, both by Northern analysis and reverse-transcriptase polymerase chain reaction.50 Caveolin-2, which is co-expressed and co-oligomerizes with caveolin-1 in most tissues, is also present in astrocytes.51
The functions of caveolin-1 and caveolae in astrocytes are consistent with what is known in other cell types: they participate in signaling and trafficking. Studies specifically identifying caveolar signaling in astrocytes include biochemical evidence that endothelin-1 mitogenic signaling leading to FAK and ERK activation is localized in caveolae.52 In addition, reactive oxygen species-induced activation of ERK in astrocytes was shown to require caveolin-1 expression.53 Calcium signaling molecules, such as TRPC1, IP3R2, and ryanodine receptor, were co-fractionated with caveolin-1 in astrocytes detergent insoluble fractions.54 Another example is interleukin beta receptor type I (IL-1RI)/Toll-like receptor type 4 (TLR4), shown to be recruited to caveolae after activation and to initiate downstream signaling in caveolae.55 Of interest, this pathway can be activated by ethanol and may mediate ethanol-induced inflammatory damage in the brain.55 In astrocytes, caveolin-1 was also documented to compartmentalize and interact with multidrug resistance protein -1 (MDR1), also named P-glycoprotein-1 (P-gp), which participates in the blood-brain barrier (BBB) function.56
Caveolae have also been proven to play trafficking functions in primary cultured astrocytes.57 Albumin endocytosis was localized to caveolae by electron microscopy and required the expression of caveolin-1.58 Caveolae also internalize the CCR2 receptor-bound ligand MCP-1,59 and caveolin-1 siRNA reduced calcium influx and astrocyte chemotaxis to MCP-1.60 Caveolin-1 further regulates cell surface expression and endocytosis of the excitatory amino acid carrier 1 (EAAC1) glutamate transporter in neuron/astrocytes mixed cultures.61 Caveolin-1 is known to participate in cholesterol trafficking. Accordingly, in astrocytes, caveolin-1 was shown to participate in cholesterol efflux to generate HDL62 and to mediate the retrograde cholesterol transport from the plasma membrane to the Golgi in response to amyloid beta protein.63
Little is known about the regulation of caveolin-1 expression in astrocytes. A study determined that a single dose of gamma ray irradiation increased caveolin-1 expression in spinal cord astrocytes 1 day after irradiation.64 In another study, primary astrocytes isolated from the striatum underwent caveolin-1 down-regulation at the protein and mRNA levels in response to cAMP and TGF-α. TGF-α–induced downregulation of caveolin-1 expression was MAP kinase-independent but PI3-kinase dependent. Furthermore, this regulation was restricted to the striatum because it was not observed in astrocytes from other brain regions. The authors hypothesized that downregulation of caveolin-1 was part of a feedback regulatory loop involving PI3K, promoting the expression of the glutamate transporter GLT-1 during astrocyte differentiation.51
Caveolin-1 and Caveolae in GBM Cells
The literature widely describes an increased expression of caveolin-1 in GBM (Table 1). However, some nuances must be kept in mind when looking at these results. First, GBMs are a mix of tumor and infiltrating nontumor cells.65 GBM lysates are likely to contain abundant caveolin-1–expressing cells (microglia, macrophages, and endothelial cells, in addition to GBM cells), compared with neurons in normal brain tissue, known to express very little caveolin-1. Moreover, there seems to be heterogeneity in caveolin-1 expression between tumor cells in each GBM, indicating that caveolin-1–positive and negative tumor cells coexist in GBM.66,67
Table 1.
Caveolin-1 Expression in Glioblastoma Multiforme
| Family member tested | Method | Tested sample | Control | Variation observed | Amplitude of the variation | Reference |
|---|---|---|---|---|---|---|
| Caveolin-1 | DNA microarray | Pooled mRNA from 2 GBM | Normal brain tissue mRNA, pooled | Increased | 5.2-fold | 68 |
| Caveolin-1 | Northern blotting and immunoblotting | mRNA and protein lysates from C6 and DITNC1 transformed astroglial cell lines | Rat type 1 primary astrocytes | Increased in C6 but not DITNC1 transformed astrocyte cell lines compared to astrocytes | 2-fold (mRNA) and 1.5-fold (protein) increased in C6 cell line | 70 |
| Caveolin-1, 2, 3 | RT-PCR and Q-PCR | mRNA from GBM cell line U87MG | fetal primary astrocytes | Increased caveolin-1 mRNA in GBM but reduced caveolin-2 and caveolin-3 | ∼1.5-fold increase (cav-1 mRNA) and 2+ fold decrease (caveolin-2 and 3 mRNA) | 69 |
| Caveolin-1 | Q-PCR | mRNA from 6 surgically removed GBM | Two samples of non-malignant brain lesions | Increased caveolin-1 mRNA in GBM tissue | 20–300 fold difference | 69 |
| Caveolin-1 | IHC | 19 GBM | Astrocytoma II and III, normal brain tissue | Increased caveolin-1 immunoreactivity associated with tumor grade - Increased plasma membrane localization in GBM | N/A | 66 |
| Caveolin-1 | Confocal immunofluorescence microscopy and FACS | U251 MG GBM cell line and 4 primary glioma cultures | Punctate staining of caveolin-1at the plasma membrane in primary GBM cells and GBM cell line reminiscent of caveolae | Significantly more caveolin-1 in GBM compared with grade III astrocytoma | 66 | |
| Caveolin-1 | Western blot analysis of tissue lysates | 9 GBM | Normal brain tissue | No statistically significant change in caveolin-1 expression by analysis of variance. | One of the GBM expresses very high amounts of caveolin-1 but not the 8 others. | 104 |
| Caveolin-1 | Immuno-histochemistry | 73 gliomas including 20 GBM | Other gliomas (low-grade astrocytomas, oligodendrogliomas…) | Variability in caveolin-1 staining both in intensity and in quantity of stained cells | Caveolin-1 expressed in more than 50% of the cells in high-grade tumors. Caveolin-1 is expressed in less than 50% of the cells in low-grade tumors. | 67 |
Abbreviation: GBM, glioblastomas.
Identification of differentially expressed genes in human GBM comparing RNA pooled from 2 GBMs to RNA pooled from normal human brain identified a 5.2-fold overexpression of caveolin-1 mRNA in GBM.68 Similarly, caveolin-1 mRNA was found to be increased in the GBM cell line U87MG, compared with primary human astrocytes, and in tumors, compared with nonmalignant brain tissue.69 In contrast, caveolin-2 and -3 mRNA were reported to be decreased in a GBM cell line, compared with primary astrocytes.69 In another study, caveolin-1 immunoreactivity was detected in astrocytic-derived tumors, with very intense staining in GBMs, whereas no immunoreactivity was detected in normal brain glia or neurons. Both staining pattern (membrane vs intracellular) and intensity were associated with tumor grade. Immunofluorescence analysis and flow cytometry confirmed with glioma cell lines and primary cultures the results observed in tissue sections.66
In various human GBM-derived cell lines (T98G, U87MG, U118MG, U138MG, and U373MG) and in rat primary astrocytes and transformed astroglial cell lines (C6 and DITNC1) abundant caveolin-1 mRNA and protein levels were detected, although with variable levels. Nucleotide sequence analysis further indicated that no mutations were present in any of the cell types.70 Of importance, caveolin-1 low-buoyant density and caveola formation were shown to be maintained.70 Caveolae were still apparent when the cells were grown ectopically in mice.70 Accordingly, caveolae have also been reported after electron microscopy observation of GBM specimens.71 Caveolae endocytosis has been proposed as a route of entry into GBM cells (T98G) for uptake of therapeutic siRNA dendriplexes, further indicating that caveolae in GBM cells are functional, and that they may be taken advantage of for therapeutic delivery.72
The published information about caveolin-1 increased expression in GBM is recapitulated in Table 1. We found no literature on quantification of caveolin-1 tyrosine 14 phosphorylation in GBM or on the expression of the caveolae-forming protein, PTRF/cavin-1, in GBM.
Antitumor Therapies of Glioma Are Associated With/Act Via Upregulation of Caveolin-1
An increased expression of caveolin-1 has been shown to be associated with and/or mediate the action of some anti-glioma therapies. One such therapeutic class are the inhibitors of the catalytic light chain (xCT) of system xc-, a Na+-independent heterodimeric aminoacid transport system that allows cystine uptake in exchange for glutamate release. Glioma cells produce high concentrations of glutamate using this transporter, which causes toxicity to healthy neurons surrounding the tumor. Furthermore, because glioma cells rely on this transporter for cystine uptake, inhibition of system xc- causes intracellular glutathione depletion resulting in apoptosis.73 In tumor cells, xCT inhibition was shown to cause reactive oxygen species–mediated caveolin-1 upregulation and inhibition of tumor cell invasion.74 Another example is the alkylating agent temozolomide, indicated in GBM treatment. Temozolomide also increased caveolin-1 expression in experimental GBM grown in vivo.75 Of interest, the authors showed that caveolin-1 exogenously applied to GBM cells decreased invasion.75 Caveolin-1 expression was also increased in glioma cells exposed to ionizing radiation;76 however, in this study, caveolin-1 was proposed to be upregulated in a transcriptionally independent fashion in multiple cell types in response to genotoxic stress and to participate in DNA damage repair.
One group of investigators has endeavored to directly manipulate caveolin-1 expression levels in the cell line U87MG and tested the resulting phenotype of the cells and their changes in gene expression. They showed that forced expression of caveolin-1 decreased proliferation, clonogenicity, and invasion, whereas downregulation of caveolin-1 had the opposite effect.77 Gene expression analysis in this study indicated that caveolin-1 controlled genes involved in the regulation of cell invasion and metastasis, for example, the serine protease inhibitor PAI-1 (inversely proportional to caveolin-1) and genes involved in the regulation of cell adhesion, such as integrins (also inversely proportional to caveolin-1 expression).77 The study further focused on integrin α5β1 and showed that GBM cells with caveolin-1 siRNA (and, thus, increased α5) were more sensitive to the α5β1 integrin antagonist SJ749. Furthermore, a feedback regulation was suggested, because overexpression of the α5 subunit in turn upregulated caveolin-1 expression.77
EGFR and Caveolin-1 in GBM
The EGFR is proposed to bind to caveolin-1 via its caveolin-1 binding motif located in the kinase domain33 and to be maintained in an inactive state by the interaction, moving out of caveolae upon EGF stimulation.78 The migration or EGFR out of caveolae requires the autophosphorylation of at least one tyrosine residue in the receptor regulatory domain and the binding of EGF.78 Several major mutated variants of EGFR have been identified in GBM, the most common being variant III (EGFRvIII), caused by an 801 bp in frame deletion of exons 2–7, leading to the expression of a constitutively autophosphorylated receptor lacking a portion of the EGF binding domain that contributes to an aggressive phenotype in GBM.79 In GBM cells, wild-type EGFR was confirmed to colocalize with lipid rafts and caveolin-1 and to associate with caveolae in a phosphorylation-dependent fashion, because EGF-induced phosphorylation of the receptor resulted in EGFR dissociation from caveolae.69 In contrast, constitutively active EGFRvIII was predominantly cytoplasmic and did not associate with the caveolin-1 scaffolding domain unless cells were exposed to a tyrosine kinase inhibitor to prevent receptor phosphorylation.69 Furthermore, disrupting rafts induced ligand-independent tyrosine phosphorylation of EGFR.69 Phosphorylation-dependent sequestration of EGFR in caveolae was thus proposed to have the potential to shut down constitutive or EGF-induced signaling in GBM.69 Of interest, low caveolin-1 expression was shown to correlate with EGFR overexpression in anaplastic astrocytomas.67 In contrast, caveola disruption using methyl-β cyclodextrin was shown to disrupt GBM chemotaxis to EGF,80 and simvastatin prevented astrocyte activation after traumatic brain injury by decreasing caveolin-1 expression and reducing EGFR phosphorylation.81
Matrix Proteases and Caveolae in GBM
Proteolysis is an essential component of the invasion process through the disruption of basement membrane, extracellular matrix, and cell-cell junctions. Two major proteolytic systems, namely the urokinase and the matrix metalloproteinase (MMP) systems, have been documented to promote GBM invasion. Furthermore, enzymes from both systems have been shown to synergistically interact in GBM.82,83
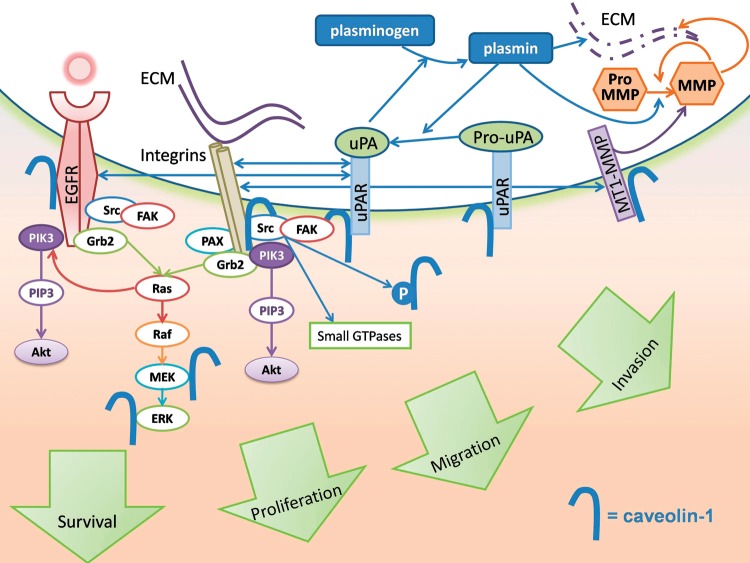
The receptor for urokinase-type plasminogen activator (uPAR) both participates in the activation of uPA, which promotes invasion and angiogenesis via plasminogen activation and further controls intracellular signaling, integrin activation, and fibronectin polymerization. uPAR was reported to be overexpressed in GBM.84 Caveolin-1 is considered to be an adaptor proteinregulating integrin function85,86 that mediates uPAR-dependent activation of Src and EGFR.87,88 Both caveolin-1 and the GPI-anchored urokinase receptor uPAR interact with β-1 integrins in a complex that regulates adhesion and signaling through Src-family kinases and focal adhesion kinase (FAK). The formation of such functional units contributes to migration and invasion.89 In GBM, uPAR controls invasion via proteolysis and key intracellular signaling pathways, including PI3k/Akt and Notch signaling.90,91 Not surprisingly, uPA and/or uPAR are well-known therapeutic targets in GBM. In addition, the crosstalk between uPAR signaling and EGFR signaling suggests that uPAR and EGFR are valid combined targets for GBM therapy.92 The role of caveolin-1 in major signaling pathways relevant to GBM is depicted in Fig. 1.
Fig. 1.
Multiple signaling pathways essential to GBM growth and invasion are controlled by caveolin-1. Receptor tyrosine kinases (EGFR), integrins, and uPA are concentrated in caveolae and interact with caveolin-1. Downstream signaling is initiated in caveolae. Caveolin-1 undergoes Src family kinase-mediated tyrosine phosphorylation upon EGF stimulation or integrin engagement. Caveolae endocytosis further controls EGFR presence at the cell surface. Binding to the caveolin-1 scaffolding domain inhibits and sequesters EGFR, as well as MT-1 MMP in caveolae. Caveolin-1 also directly inhibits MEK-1 and ERK-2 downstream of the EGFR activation. UPA forms a complex with integrins and caveolin-1 that signals through Src family kinases and focal adhesion kinase (FAK). Via interacting with the uPA receptor and MT1-MMP, caveolin-1 spatially controls extracellular proteolysis, including growth factor release from the extracellular matrix, and basement membrane degradation.
Matrix metalloproteases are a family of endopeptidases mediating pericellular proteolysis in tumor cell invasion though digestion of the extracellular matrix (ECM), release of ECM growth factors, and interaction with adhesion molecules, thereby controlling the tumor microenvironment.65 MMPs exist as secreted latent soluble forms activated by proteolysis, for example, the gelatinases MMP-2 and MMP-9, and as membrane-inserted enzymes, for example, membrane type MT1-MMP.93 MT1-MMP degrades ECM molecules, including constituents of the brain and glioma ECM, and further activates pro-MMP2 into MMP2.93 Both quantitative expression of MT1-MMP94 and its subcellular localization95 have been documented to play a key role in GBM cell migration and invasion. MT1-MMP is localized in caveolae and interacts with phosphocaveolin-1.96 Overexpression of caveolin-1 in GBM cells markedly represses MT1-MMP–dependent migration.95
Caveolin-1 Negatively Regulates P-gp: Potential Role in Preventing Multidrug Resistance
The permeability glycoprotein (P-gp) transporter is an efflux pump that participates in multidrug resistance by promoting efflux of xenobiotics and, thus, decreasing drug accumulation in the cells. It is highly expressed by endothelial cells and astrocytes at the BBB,97–99 including the BBB of brain tumors,100 where it can act to prevent drugs from reaching the brain. In addition, it is expressed by multidrug-resistant tumor cells. Brain tumor cells were shown to express P-gp, although at a lower level than the tumor capillaries.100 Immunolabeling and biochemical assays have demonstrated that, in various cell types including astrocytes, a proportion of cell membrane P-gp associates with caveolae, where it interacts with the caveolin-1 scaffolding domain.56,97 The interaction with caveolin-1 negatively regulates P-gp transport activity, thereby promoting intracellular transport of drugs.97 In this context, increasing caveolin-1–mediated inhibition of P-gp in GBM cells might increase sensitivity to chemotherapeutic agents.
Conclusion and Perspectives
There is increasing evidence that caveolin-1 and caveolae play multiple signaling and trafficking functions in GBM marking this organelle and/or its defining protein as targets for a disease that desperately needs novel therapeutic options. Caveolae have been scrutinized as drug delivery routes, and because GBM's caveolae might be used to promote endocytosis of material with potential therapeutic value to cells (eg, pharmacological agents and genetic material). Demonstrated uptake of therapeutic siRNA dendriplexes in GBM cells72 provides promising proof of concept for this strategy. In addition, the caveolin-1 inhibitory effect on P-gp has the potential to increase drug delivery to GBM cells via action on the transporter at the BBB and GBM cell levels.
Both increasing and decreasing caveolin-1 expression have been proposed as cancer therapeutic approaches,9 using ectopic expression of caveolin-1 or siRNA, respectively. In GBM, caveolin-1 overexpression rather than downregulation induced a reduction in proliferation, clonogenicity, and migration.77 This may seem counterintuitive, as caveolin-1 is reported to be overexpressed in GBM compared with normal astrocytes. However, caveolin-1 exerts multiple effects (direct inhibition, receptor endocytosis from cell surface, and compartmentalization) on multiple signaling pathways key to GBM growth and invasion. Another means of taking advantage of caveolin-1 tonic inhibition on signaling molecules that has proven to be effective in vivo is to mimic the inhibitory effect by using the caveolin-1 scaffolding domain peptide, fused to an internalization sequence allowing cellular uptake.101 Although initially designed to control nitric oxide production in endothelial cells, this peptide, named cavtratin, was shown to suppress nerve growth factor–induced MAPK activation in oligodendrocytes, indicating that inhibition of growth factor receptors can be achieved in macroglial cells.102 Mutations of specific amino acids in the scaffolding domain peptide can perturb, rather than reproduce, the interaction with target signaling molecules,103 and although this has been worked out for the interaction of caveolin-1 with eNOS, the concept could be applied to generate peptides affecting growth factor receptor inhibition. Lastly, an indirect method to modulate caveolin-1 expression and caveolae function is to interfere with cholesterol homeostasis with use of statins. Of interest, simvastatin decreased caveolin-1 expression and reduced EGFR phosphorylation in astrocytes.81 It is likely that caveolin-1 will be evaluated as a target in GBM in the near future, alone or in combination with currently available therapies to increase their efficacy.
Conflict of interest statement. M-O.P and G.J.R.: no reported conflicts.
Funding
M-O.P. is funded by the Cancer Council Queensland Research Grant 631368. G.J.R. is funded by the Virginia and D.K. Ludwig Fund for Cancer Research and the Irving J. Sherman M.D. Neurosurgery Research Professorship.
References
- 1.Parat MO. The biology of caveolae: achievements and perspectives. Int Rev Cell Mol Biol. 2009;273:117–162. doi: 10.1016/S1937-6448(08)01804-2. [DOI] [PubMed] [Google Scholar]
- 2.Hill MM, Bastiani M, Luetterforst R, et al. PTRF-Cavin, a Conserved Cytoplasmic Protein Required for Caveola Formation and Function. Cell. 2008;132(1):113–124. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayer A, Stoeber M, Bissig C, Helenius A. Biogenesis of Caveolae: Stepwise Assembly of Large Caveolin and Cavin Complexes. Traffic. 2010;11(3):361–382. doi: 10.1111/j.1600-0854.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- 4.Dietzen DJ, Hastings WR, Lublin DM. Caveolin is palmitoylated on multiple cysteine residues. Palmitoylation is not necessary for localization of caveolin to caveolae. J Biol Chem. 1995;270:6838–6842. doi: 10.1074/jbc.270.12.6838. [DOI] [PubMed] [Google Scholar]
- 5.Epand RM, Sayer BG, Epand RF. Caveolin Scaffolding Region and Cholesterol-rich Domains in Membranes. Journal of Molecular Biology. 2005;345(2):339–350. doi: 10.1016/j.jmb.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 6.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin- scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 7.Schlegel A, Arvan P, Lisanti MP. Caveolin-1 binding to endoplasmic reticulum membranes and entry into the regulated secretory pathway are regulated by serine phosphorylation. Protein sorting at the level of the endoplasmic reticulum. J Biol Chem. 2001;276(6):4398–4408. doi: 10.1074/jbc.M005448200. [DOI] [PubMed] [Google Scholar]
- 8.Glenney JR., Jr Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J Biol Chem. 1989;264:20163–20166. [PubMed] [Google Scholar]
- 9.van Golen KL. Is caveolin-1 a viable therapeutic target to reduce cancer metastasis? Expert Opin Ther Targets. 2006;10(5):709–721. doi: 10.1517/14728222.10.5.709. [DOI] [PubMed] [Google Scholar]
- 10.Thompson TC, Tahir SA, Li L, et al. The role of caveolin-1 in prostate cancer: clinical implications. Prostate Cancer Prostatic Dis. 2010;13(1):6–11. doi: 10.1038/pcan.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288(3):C494–C506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- 12.Goetz J, Lajoie P, Wiseman S, Nabi I. Caveolin-1 in tumor progression: the good, the bad and the ugly. Cancer and Metastasis Reviews. 2008;27(4):715–735. doi: 10.1007/s10555-008-9160-9. [DOI] [PubMed] [Google Scholar]
- 13.Engelman JA, Zhang XL, Lisanti MP. Genes encoding human caveolin-1 and -2 are co-localized to the D7S522 locus (7q31.1), a known fragile site (FRA7G) that is frequently deleted in human cancers. FEBS Lett. 1998;436:403–410. doi: 10.1016/s0014-5793(98)01134-x. [DOI] [PubMed] [Google Scholar]
- 14.Tahir SA, Ren C, Timme TL, et al. Development of an immunoassay for serum caveolin-1: a novel biomarker for prostate cancer. Clin Cancer Res. 2003;9(10, pt 1):3653–3659. [PubMed] [Google Scholar]
- 15.Tahir SA, Yang G, Ebara S, et al. Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen-insensitive prostate cancer. Cancer Res. 2001;61(10):3882–3885. [PubMed] [Google Scholar]
- 16.Tahir SA, Yang G, Goltsov AA, et al. Tumor cell-secreted caveolin-1 has proangiogenic activities in prostate cancer. Cancer Res. 2008;68(3):731–739. doi: 10.1158/0008-5472.CAN-07-2668. [DOI] [PubMed] [Google Scholar]
- 17.Goetz JG, Minguet S, Navarro-Lerida I, et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146(1):148–163. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill MM, Parat MO, Parton RG, Soon Lee C. Clearing the confusion of Cav1 and caveolae in cancer compartments. Cell. 2011;146(1):148–163. Comment on: Cell http://www.cell.com/abstract/S0092-8674%2811%2900645-3#Comments. 2011. Ref Type: Internet Communication. [Google Scholar]
- 19.Aung CS, Hill MM, Bastiani M, Parton RG, Parat MO. PTRF-cavin-1 expression decreases the migration of PC3 prostate cancer cells: Role of matrix metalloprotease 9. Eur J Cell Biol. 2010;90:136–142. doi: 10.1016/j.ejcb.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Steffens S, Schrader A, Blasig H, et al. Caveolin 1 protein expression in renal cell carcinoma predicts survival. BMC Urology. 2011;11(1):25. doi: 10.1186/1471-2490-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastiani M, Liu L, Hill MM, et al. MURC/Cavin-4 and cavin family members form tissue-specific caveolar complexes. J Cell Biol. 2009;185(7):1259–1273. doi: 10.1083/jcb.200903053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansa P, Grummt I. Mechanism of transcription termination: PTRF interacts with the largest subunit of RNA polymerase I and dissociates paused transcription complexes from yeast and mouse. Mol Gen Genet. 1999;262(3):508–514. doi: 10.1007/s004380051112. [DOI] [PubMed] [Google Scholar]
- 23.Jansa P, Mason SW, Hoffmann-Rohrer U, Grummt I. Cloning and functional characterization of PTRF, a novel protein which induces dissociation of paused ternary transcription complexes. EMBO J. 1998;17(10):2855–2864. doi: 10.1093/emboj/17.10.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasegawa T, Takeuchi A, Miyaishi O, Xiao H, Mao J, Isobe K. PTRF (polymerase I and transcript-release factor) is tissue-specific and interacts with the BFCOL1 (binding factor of a type-I collagen promoter) zinc-finger transcription factor which binds to the two mouse type-I collagen gene promoters. Biochem J. 2000;347(pt 1):55–59. [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen CG, Bright NA, Howard G, Nichols BJ. SDPR induces membrane curvature and functions in the formation of caveolae. Nat Cell Biol. 2009;11(7):807–814. doi: 10.1038/ncb1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMahon KA, Zajicek H, Li WP, et al. SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. EMBO J. 2009;28(8):1001–1015. doi: 10.1038/emboj.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai L, Deng X, Li Q, et al. Down-regulation of the cavin family proteins in breast cancer. J Cell Biochem. 2012;113(1):322–328. doi: 10.1002/jcb.23358. [DOI] [PubMed] [Google Scholar]
- 28.Gould ML, Williams G, Nicholson HD. Changes in caveolae, caveolin, and polymerase 1 and transcript release factor (PTRF) expression in prostate cancer progression. Prostate. 2010;70(15):1609–1621. doi: 10.1002/pros.21195. [DOI] [PubMed] [Google Scholar]
- 29.Inder KL, Zheng YZ, Davis MJ, et al. Expression of PTRF in PC-3 cells modulates cholesterol dynamics and the actin cytoskeleton impacting secretion pathways. Molecular & Cellular Proteomics. 2012;11(1):119–131. doi: 10.1074/mcp.M111.012245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen WL, Stevenson L, Coyle VM, et al. A Systems Biology Approach Identifies SART1 as a Novel Determinant of Both 5-Fluorouracil and SN38 Drug Resistance in Colorectal Cancer. Molecular Cancer Therapeutics. 2012;11(2):M111–012245. doi: 10.1158/1535-7163.MCT-11-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guha U, Chaerkady R, Marimuthu A, et al. Comparisons of tyrosine phosphorylated proteins in cells expressing lung cancer-specific alleles of EGFR and KRAS. PNAS. 2008;105(37):14112–14117. doi: 10.1073/pnas.0806158105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mineo C, James GL, Smart EJ, Anderson RG. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem. 1996;271:11930–11935. doi: 10.1074/jbc.271.20.11930. [DOI] [PubMed] [Google Scholar]
- 33.Couet J, Sargiacomo M, Lisanti MP. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem. 1997;272:30429–30438. doi: 10.1074/jbc.272.48.30429. [DOI] [PubMed] [Google Scholar]
- 34.Engelman JA, Chu C, Lin A, et al. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett. 1998;428:205–211. doi: 10.1016/s0014-5793(98)00470-0. [DOI] [PubMed] [Google Scholar]
- 35.Ringerike T, Blystad FD, Levy FO, Madshus IH, Stang E. Cholesterol is important in control of EGF receptor kinase activity but EGF receptors are not concentrated in caveolae. J Cell Sci. 2002;115(6):1331–1340. doi: 10.1242/jcs.115.6.1331. [DOI] [PubMed] [Google Scholar]
- 36.Sbaa E, Frerart F, Feron O. The double regulation of endothelial nitric oxide synthase by caveolae and caveolin: a paradox solved through the study of angiogenesis. Trends Cardiovasc Med. 2005;15(5):157–162. doi: 10.1016/j.tcm.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Hill MM, Scherbakov N, Schiefermeier N, et al. Reassessing the role of phosphocaveolin-1 in cell adhesion and migration. Traffic. 2007;8(12):1695–1705. doi: 10.1111/j.1600-0854.2007.00653.x. [DOI] [PubMed] [Google Scholar]
- 38.Parat MO, Anand-Apte B, Fox PL. Differential caveolin-1 polarization in endothelial cells during migration in two and three dimensions. Mol Biol Cell. 2003;14:3156–3168. doi: 10.1091/mbc.E02-11-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santilman V, Baran J, Anand-Apte B, Evans RM, Parat MO. Caveolin-1 polarization in transmigrating endothelial cells requires binding to intermediate filaments. Angiogenesis. 2007;10(4):297–305. doi: 10.1007/s10456-007-9083-z. [DOI] [PubMed] [Google Scholar]
- 40.Lee H, Volonte D, Galbiati F, et al. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav- 1/Grb7 signaling cassette. Mol Endocrinol. 2000;14(11):1750–1775. doi: 10.1210/mend.14.11.0553. [DOI] [PubMed] [Google Scholar]
- 41.Cao H, Courchesne WE, Mastick CC. A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14: recruitment of C-terminal Src kinase. J Biol Chem. 2002;277(11):8771–8774. doi: 10.1074/jbc.C100661200. [DOI] [PubMed] [Google Scholar]
- 42.Gaus K, Le LS, Balasubramanian N, Schwartz MA. Integrin-mediated adhesion regulates membrane order. J Cell Biol. 2006;174(5):725–734. doi: 10.1083/jcb.200603034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goetz JG, Joshi B, Lajoie P, et al. Concerted regulation of focal adhesion dynamics by galectin-3 and tyrosine-phosphorylated caveolin-1. J Cell Biol. 2008;130(6):1261–1273. doi: 10.1083/jcb.200709019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radel C, Rizzo V. Integrin mechanotransduction stimulates caveolin-1 phosphorylation and recruitment of Csk to mediate actin reorganization. Am J Physiol Heart Circ Physiol. 2005;288(2):H936–H945. doi: 10.1152/ajpheart.00519.2004. [DOI] [PubMed] [Google Scholar]
- 45.del Pozo MA, Balasubramanian N, Alderson NB, et al. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7(9):901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galbiati F, Volonte D, Gil O, et al. Expression of caveolin-1 and -2 in differentiating PC12 cells and dorsal root ganglion neurons: caveolin-2 is up-regulated in response to cell injury. Proc Natl Acad Sci USA. 1998;95:10257–10262. doi: 10.1073/pnas.95.17.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Massa PT. Plasmalemmal vesicles (caveolae) of fibrous astrocytes of the cat optic nerve. Am J Anat. 1982;165(1):69–81. doi: 10.1002/aja.1001650107. [DOI] [PubMed] [Google Scholar]
- 48.Lawrence JM, Raisman G. Membrane specializations and extracellular material associated with host astrocytes in peripheral neural transplants. Neuroscience. 1987;20(3):1031–1041. doi: 10.1016/0306-4522(87)90261-2. [DOI] [PubMed] [Google Scholar]
- 49.Massa PT, Mugnaini E. Cell-cell junctional interactions and characteristic plasma membrane features of cultured rat glial cells. Neuroscience. 1985;14(2):695–709. doi: 10.1016/0306-4522(85)90320-3. [DOI] [PubMed] [Google Scholar]
- 50.Cameron PL, Ruffin JW, Bollag R, Rasmussen H, Cameron RS. Identification of caveolin and caveolin-related proteins in the brain. J Neurosci. 1997;17(24):9520–9535. doi: 10.1523/JNEUROSCI.17-24-09520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zschocke J, Bayatti N, Behl C. Caveolin and GLT-1 gene expression is reciprocally regulated in primary astrocytes: Association of GLT-1 with non-caveolar lipid rafts. Glia. 2005;49(2):275–287. doi: 10.1002/glia.20116. [DOI] [PubMed] [Google Scholar]
- 52.Teixeira A, Chaverot N, Schroder C, Strosberg AD, Couraud PO, Cazaubon S. Requirement of caveolae microdomains in extracellular signal-regulated kinase and focal adhesion kinase activation induced by endothelin-1 in primary astrocytes. J Neurochem. 1999;72:120–128. doi: 10.1046/j.1471-4159.1999.0720120.x. [DOI] [PubMed] [Google Scholar]
- 53.Yun JH, Park SJ, Jo A, et al. Caveolin-1 is involved in reactive oxygen species-induced SHP-2 activation in astrocytes. Exp Mol Med. 2011;43(12):660–668. doi: 10.3858/emm.2011.43.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weerth SH, Holtzclaw LA, Russell JT. Signaling proteins in raft-like microdomains are essential for Ca2+ wave propagation in glial cells. Cell Calcium. 2007;41(2):155–167. doi: 10.1016/j.ceca.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Blanco AM, Perez-Arago A, Fernandez-Lizarbe S, Guerri C. Ethanol mimics ligand-mediated activation and endocytosis of IL-1RI/TLR4 receptors via lipid rafts caveolae in astroglial cells. Journal of Neurochemistry. 2008;106(2):625–639. doi: 10.1111/j.1471-4159.2008.05425.x. [DOI] [PubMed] [Google Scholar]
- 56.Ronaldson PT, Bendayan M, Gingras D, Piquette-Miller M, Bendayan R. Cellular localization and functional expression of P-glycoprotein in rat astrocyte cultures. Journal of Neurochemistry. 2004;89(3):788–800. doi: 10.1111/j.1471-4159.2004.02417.x. [DOI] [PubMed] [Google Scholar]
- 57.Megias L, Guerri C, Fornas E, et al. Endocytosis and transcytosis in growing astrocytes in primary culture. Possible implications in neural development. Int J Dev Biol. 2000;44(2):209–221. [PubMed] [Google Scholar]
- 58.Bento-Abreu A, Velasco A, Polo-Hernández E, et al. Albumin endocytosis via megalin in astrocytes is caveola- and Dab-1 dependent and is required for the synthesis of the neurotrophic factor oleic acid. Journal of Neurochemistry. 2009;111(1):49–60. doi: 10.1111/j.1471-4159.2009.06304.x. [DOI] [PubMed] [Google Scholar]
- 59.Andjelkovic AV, Song L, Dzenko KA, Cong H, Pachter JS. Functional expression of CCR2 by human fetal astrocytes. Journal of Neuroscience Research. 2002;70(2):219–231. doi: 10.1002/jnr.10372. [DOI] [PubMed] [Google Scholar]
- 60.Ge S, Pachter JS. Caveolin-1 knockdown by small interfering RNA suppresses responses to the chemokine MCP-1 by human astrocytes. J Biol Chem. 2004;279(8):6688–6695. doi: 10.1074/jbc.M311769200. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez MI, Krizman-Genda E, Robinson MB. Caveolin-1 Regulates the Delivery and Endocytosis of the Glutamate Transporter, Excitatory Amino Acid Carrier 1. Journal of Biological Chemistry. 2007;282(41):29855–29865. doi: 10.1074/jbc.M704738200. [DOI] [PubMed] [Google Scholar]
- 62.Ito J, Nagayasu Y, Kato K, Sato R, Yokoyama S. Apolipoprotein A-I Induces Translocation of Cholesterol, Phospholipid, and Caveolin-1 to Cytosol in Rat Astrocytes. Journal of Biological Chemistry. 2002;277(10):7929–7935. doi: 10.1074/jbc.M103878200. [DOI] [PubMed] [Google Scholar]
- 63.Igbavboa U, Sun GY, Weisman GA, He Y, Wood WG. Amyloid beta-protein stimulates trafficking of cholesterol and caveolin-1 from the plasma membrane to the Golgi complex in mouse primary astrocytes. Neuroscience. 2009;162(2):328–338. doi: 10.1016/j.neuroscience.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahn M, Kim H, Kim JT, et al. Gamma-ray irradiation stimulates the expression of caveolin-1 and GFAP in rat spinal cord: a study of immunoblot and immunohistochemistry. J Vet Sci. 2006;7(4):309–314. doi: 10.4142/jvs.2006.7.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alves TR, Lima FRS, Kahn SA, et al. Glioblastoma cells: A heterogeneous and fatal tumor interacting with the parenchyma. Life Sciences. 2011;89(15–16):532–539. doi: 10.1016/j.lfs.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 66.Cassoni P, Senetta R, Castellano I, et al. Caveolin-1 Expression is Variably Displayed in Astroglial-derived Tumors and Absent in Oligodendrogliomas: Concrete Premises for a New Reliable Diagnostic Marker in Gliomas. The American Journal of Surgical Pathology. 2007;31(5):760–769. doi: 10.1097/01.pas.0000213433.14740.5d. [DOI] [PubMed] [Google Scholar]
- 67.Barresi V, Buttarelli FR, Vitarelli E E, Arcella A, Antonelli M, Giangaspero F. Caveolin-1 expression in diffuse gliomas: correlation with the proliferation index, epidermal growth factor receptor, p53, and 1p/19q status. Human Pathology. 2009;40(12):1738–1746. doi: 10.1016/j.humpath.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 68.Sallinen SL, Sallinen PK, Haapasalo HK, et al. Identification of differentially expressed genes in human gliomas by DNA microarray and tissue chip techniques. Cancer Res. 2000;60(23):6617–6622. [PubMed] [Google Scholar]
- 69.Abulrob A, Giuseppin S, Andrade MF, McDermid A, Moreno M, Stanimirovic D. Interactions of EGFR and caveolin-1 in human glioblastoma cells: evidence that tyrosine phosphorylation regulates EGFR association with caveolae. Oncogene. 2004;23(41):6967–6979. doi: 10.1038/sj.onc.1207911. [DOI] [PubMed] [Google Scholar]
- 70.Cameron PL, Liu C, Smart DK, Hantus ST, Fick JR, Cameron RS. Caveolin-1 expression is maintained in rat and human astroglioma cell lines. Glia. 2002;37(3):275–290. doi: 10.1002/glia.10036. [DOI] [PubMed] [Google Scholar]
- 71.Ludwig HC, Rausch S, Schallock K, Markakis E. Expression of CD 73 (ecto-5′-nucleotidase) in 165 glioblastomas by immunohistochemistry and electronmicroscopic histochemistry. Anticancer Res. 1999;19(3A):1747–1752. [PubMed] [Google Scholar]
- 72.Perez AP, Cosaka ML, Romero EL, Morilla MJ. Uptake and intracellular traffic of siRNA dendriplexes in glioblastoma cells and macrophages. Int J Nanomedicine. 2011;6:2715–2728. doi: 10.2147/IJN.S25235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chung WJ, Lyons SA, Nelson GM, et al. Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci. 2005;25(31):7101–7110. doi: 10.1523/JNEUROSCI.5258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen RS, Song YM, Zhou ZY, et al. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/[beta]-catenin pathway. Oncogene. 2008;28(4):599–609. doi: 10.1038/onc.2008.414. [DOI] [PubMed] [Google Scholar]
- 75.Bruyere C, Abeloos L, Lamoral-Theys D, et al. Temozolomide modifies caveolin-1 expression in experimental malignant gliomas in vitro and in vivo. Transl Oncol. 2011;4(2):92–100. doi: 10.1593/tlo.10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu H, Yue J, Pan Z, et al. Involvement of Caveolin-1 in Repair of DNA Damage through Both Homologous Recombination and Non-Homologous End Joining. PLoS ONE. 2010;5(8):e12055. doi: 10.1371/journal.pone.0012055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin S, Cosset EC, Terrand J, Maglott A, Takeda K, Dontenwill M. Caveolin-1 regulates glioblastoma aggressiveness through the control of alpha(5)beta(1) integrin expression and modulates glioblastoma responsiveness to SJ749, an alpha(5)beta(1) integrin antagonist. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2009;1793(2):354–367. doi: 10.1016/j.bbamcr.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 78.Mineo C, Gill GN, Anderson RG. Regulated Migration of Epidermal Growth Factor Receptor from Caveolae. J Biol Chem. 1999;274:30636–30643. doi: 10.1074/jbc.274.43.30636. [DOI] [PubMed] [Google Scholar]
- 79.Nishikawa R, Ji XD, Harmon RC, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. PNAS. 1994;91(16):7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bomben VC, Turner KL, Barclay TT, Sontheimer H. Transient receptor potential canonical channels are essential for chemotactic migration of human malignant gliomas. Journal of Cellular Physiology. 2011;226(7):1879–1888. doi: 10.1002/jcp.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu H, Mahmood A, Lu D, et al. Attenuation of astrogliosis and modulation of endothelial growth factor receptor in lipid rafts by simvastatin after traumatic brain injury. Journal of Neurosurgery. 2009;113(3):591–597. doi: 10.3171/2009.9.JNS09859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Le DM, Besson A, Fogg DK, et al. Exploitation of astrocytes by glioma cells to facilitate invasiveness: a mechanism involving matrix metalloproteinase-2 and the urokinase-type plasminogen activator-plasmin cascade. J Neurosci. 2003;23(10):4034–4043. doi: 10.1523/JNEUROSCI.23-10-04034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lakka SS, Gondi CS, Dinh DH, et al. Specific Interference of Urokinase-type Plasminogen Activator Receptor and Matrix Metalloproteinase-9 Gene Expression Induced by Double-stranded RNA Results in Decreased Invasion, Tumor Growth, and Angiogenesis in Gliomas. Journal of Biological Chemistry. 2005;280(23):21882–21892. doi: 10.1074/jbc.M408520200. [DOI] [PubMed] [Google Scholar]
- 84.Salajegheh M, Rudnicki A, Smith TW. Expression of urokinase-type plasminogen activator receptor (uPAR) in primary central nervous system neoplasms. Appl Immunohistochem Mol Morphol. 2005;13(2):184–189. doi: 10.1097/01.pai.0000138448.85231.da. [DOI] [PubMed] [Google Scholar]
- 85.Chapman HA, Wei Y, Simon DI, Waltz DA. Role of urokinase receptor and caveolin in regulation of integrin signaling. Thromb Haemost. 1999;82(2):291–297. [PubMed] [Google Scholar]
- 86.Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–634. doi: 10.1016/s0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- 87.Monaghan-Benson E, Keown-Longo PJ. Urokinase-type Plasminogen Activator Receptor Regulates a Novel Pathway of Fibronectin Matrix Assembly Requiring Src-dependent Transactivation of Epidermal Growth Factor Receptor. Journal of Biological Chemistry. 2006;281(14):9450–9459. doi: 10.1074/jbc.M501901200. [DOI] [PubMed] [Google Scholar]
- 88.Monaghan-Benson E, Mastick CC, Keown-Longo PJ. A dual role for caveolin-1 in the regulation of fibronectin matrix assembly by uPAR. J Cell Sci. 2008;121(22):3693–3703. doi: 10.1242/jcs.028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Preissner KT, Kanse SM, May AE. Urokinase receptor: a molecular organizer in cellular communication. Curr Opin Cell Biol. 2000;12(5):621–628. doi: 10.1016/s0955-0674(00)00141-1. [DOI] [PubMed] [Google Scholar]
- 90.Chandrasekar N, Mohanam S, Gujrati M, Olivero WC, Dinh DH, Rao JS. Downregulation of uPA inhibits migration and PI3k/Akt signaling in glioblastoma cells. Oncogene. 2003;22(3):392–400. doi: 10.1038/sj.onc.1206164. [DOI] [PubMed] [Google Scholar]
- 91.Raghu H, Gondi C, Dinh D, Gujrati M, Rao J. Specific knockdown of uPA/uPAR attenuates invasion in glioblastoma cells and xenografts by inhibition of cleavage and trafficking of Notch -1 receptor. Molecular Cancer. 2011;10(1):130. doi: 10.1186/1476-4598-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu J, Jo M, Cavenee WK, Furnari F, VandenBerg SR, Gonias SL. Crosstalk between the urokinase-type plasminogen activator receptor and EGF receptor variant III supports survival and growth of glioblastoma cells. PNAS. 2011;108(38):15984–15989. doi: 10.1073/pnas.1113416108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fillmore HL, VanMeter TE, Broaddus WC. Membrane-type Matrix Metalloproteinases (MT-MMP)s: Expression and Function During Glioma Invasion. Journal of Neuro-Oncology. 2001;53(2):187–202. doi: 10.1023/a:1012213604731. [DOI] [PubMed] [Google Scholar]
- 94.Deryugina EI, Bourdon MA, Luo GX, Reisfeld RA, Strongin A. Matrix metalloproteinase-2 activation modulates glioma cell migration. J Cell Sci. 1997;110(19):2473–2482. doi: 10.1242/jcs.110.19.2473. [DOI] [PubMed] [Google Scholar]
- 95.Annabi B, Lachambre M, Bousquet-Gagnon N, Page M, Gingras D, Beliveau R. Localization of membrane-type 1 matrix metalloproteinase in caveolae membrane domains. Biochem J. 2001;353(pt 3):547–553. doi: 10.1042/0264-6021:3530547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Labrecque L, Nyalendo C, Langlois S, et al. Src-mediated Tyrosine Phosphorylation of Caveolin-1 Induces Its Association with Membrane Type 1 Matrix Metalloproteinase. J Biol Chem. 2004;279(50):52132–52140. doi: 10.1074/jbc.M409617200. [DOI] [PubMed] [Google Scholar]
- 97.Jodoin J, Demeule M, Fenart L, et al. P-glycoprotein in blood–brain barrier endothelial cells: interaction and oligomerization with caveolins. Journal of Neurochemistry. 2003;87(4):1010–1023. doi: 10.1046/j.1471-4159.2003.02081.x. [DOI] [PubMed] [Google Scholar]
- 98.Golden PL, Pardridge WM. P-glycoprotein on astrocyte foot processes of unfixed isolated human brain capillaries. Brain Research. 1999;819(1–2):143–146. doi: 10.1016/s0006-8993(98)01305-5. [DOI] [PubMed] [Google Scholar]
- 99.Bendayan R, Ronaldson PT, Gingras D, Bendayan M. In Situ Localization of P-glycoprotein (ABCB1) in Human and Rat Brain. Journal of Histochemistry & Cytochemistry. 2006;54(10):1159–1167. doi: 10.1369/jhc.5A6870.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo Z, Zhu J, Zhao L, Luo Q, Jin X. Expression and clinical significance of multidrug resistance proteins in brain tumors. J Exp Clin Cancer Res. 2010;29:122. doi: 10.1186/1756-9966-29-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bucci M, Gratton JP, Rudic RD, et al. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6(12):1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 102.Schmitz M, Zerr I, Althaus H. Effect of Cavtratin, a Caveolin-1 Scaffolding Domain Peptide, on Oligodendroglial Signaling Cascades. Cellular and Molecular Neurobiology. 2011;31(7):991–997. doi: 10.1007/s10571-011-9694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bernatchez P, Sharma A, Bauer PM, Marin E, Sessa WC. A noninhibitory mutant of the caveolin-1 scaffolding domain enhances eNOS-derived NO synthesis and vasodilation in mice. J Clin Invest. 2011;121(9):3747–3755. doi: 10.1172/JCI44778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Forget MA, Desrosiers RR, Del Maestro RF, et al. The expression of Rho proteins decreases with human brain tumor progression: Potential tumor markers. Clinical and Experimental Metastasis. 2002;19(1):9–15. doi: 10.1023/a:1013884426692. [DOI] [PubMed] [Google Scholar]