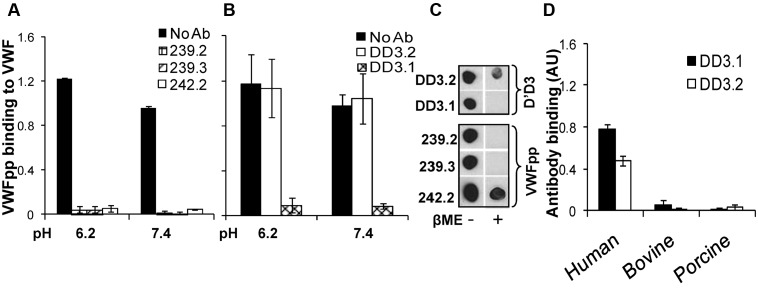
Figure 2.
Blocking VWF binding to VWFpp. (A) Anti-VWFpp (10 μg/mL) mAbs (239.2, 239.3, and 242.2) was added to microtiter wells bearing immobilized VWFpp for 15 minutes. Next, 2.5 μg/mL of VWF was added in buffer at either low pH (MES buffer, pH 6.2, with 10mM CaCl2) or high pH (HEPES buffer, pH 7.4, with 2.5mM CaCl2). Binding of multimeric VWF to VWFpp was measured using polyclonal anti-VWF Ab. (B) Studies identical to those in panel A were conducted except that 1 μg/mL of anti-D′D3 mAbs (DD3.1 and DD3.2) were incubated with plasma VWF for 15 minutes before the addition of the mixture to wells containing immobilized VWFpp. (C) Fifty nanograms of either native or denatured (heated with β-mercaptoethanol at 95°C for 5 minutes) D′D3 or VWFpp was spotted onto nitrocellulose membrane. After membrane blocking, binding of anti-D′D3 and anti-VWFpp mAbs to these spots was measured using HRP-conjugated goat anti–mouse Ab for detection. (D) Human, porcine, or bovine plasma was immobilized onto wells bearing anti-VWF polyclonal Ab such that equivalent amounts of VWF were captured in each well as determined using HRP-conjugated anti-VWF polyclonal Ab. Binding of anti-D′D3 mAbs to VWF was then measured using HRP-conjugated goat anti–mouse Ab. Neither mAb bound porcine or bovine VWF.