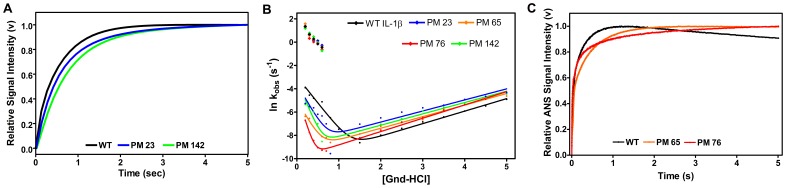
Figure 4. Representative traces of the folding kinetics and chevron plot of the relaxation rates indicating the effects of altered chain connectivity.
(A) WT IL-1β is in black, PM23 in blue, and PM142 is in green. The traces are of the first 5 seconds of stopped-flow kinetic refolding jumps from 2.2 M Gdn-HCl to 0.4 M Gdn-HCl. Kinetic curves of WT and permutant proteins were fit to a three-exponential model. (B) Plot of the natural log of kobs obtained by both stopped-flow and manual mixing refolding and unfolding experiments for all proteins variants as a function of final denaturant concentration. A comparison of the observed relaxation rates obtained by stopped-flow refolding experiments associated with the formation of the intermediate (upper left, diamonds) for WT IL-1β (black) indicating the similarities in observed rates of intermediate formation between PM23 (blue), PM65 (orange), PM76 (red), and PM142 (green). The data points (lower left and right, circles) and fits depict the rate of formation of the native protein. The differences in slope of the curves are a result of the cut site, where the greatest change in folding is seen in PM76 (red), indicating a slow transition to native. (C) ANS stopped-flow fluorescence-detected refolding of WT and permutant IL-1β. WT, in black, and permutant IL-1β protein (PM65 in orange, PM76 in red) were refolded from an unfolded state in the presence of ANS. The traces are representative of refolding jumps from 2.2 M to 0.3 M Gdn-HCl. The first 5 seconds is shown for clarity. The resulting curves confirm the similarity in the formation of the kinetic intermediate, but indicate a change in the release of the florophore.