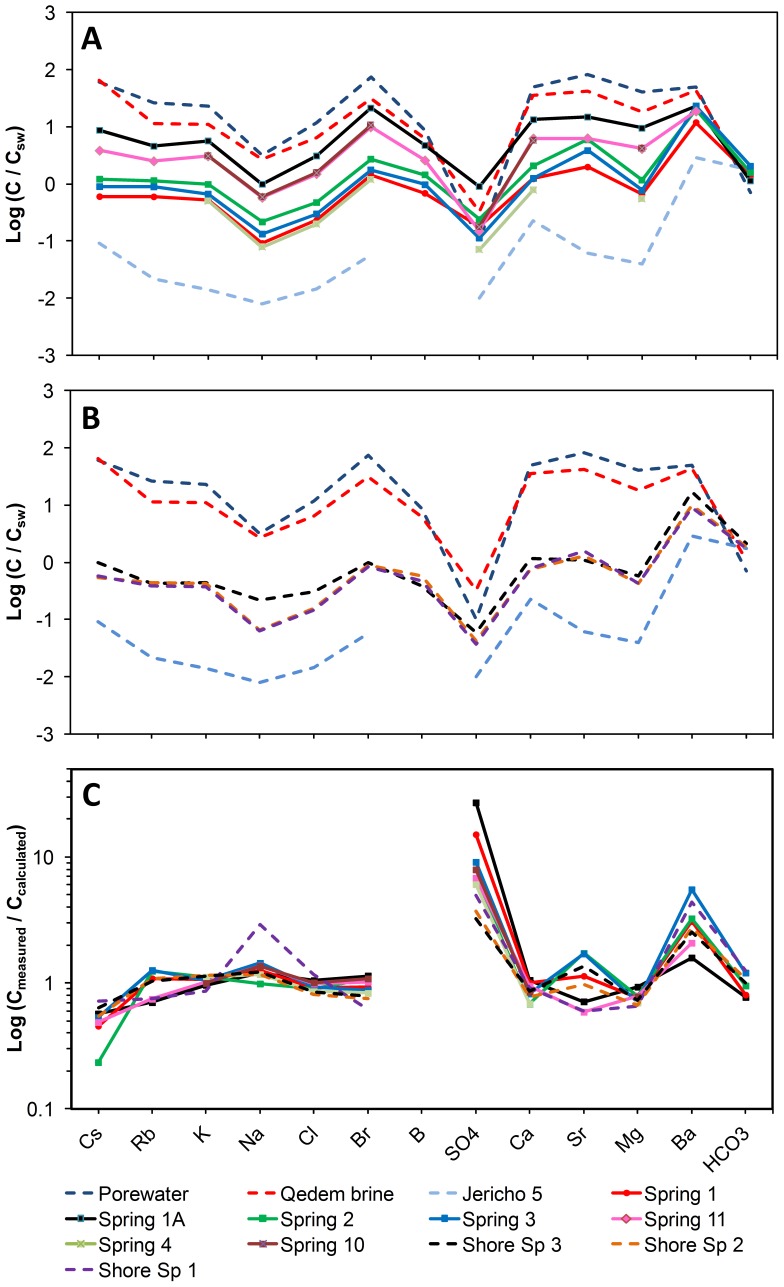
Figure 3. Seawater normalized (Csw) concentrations of major ions in waters from the underwater springs (A) and from reference sites (B).
The concentrations are listed in Table 2 and in Table S1. The ions are arranged along the x-axis based on their natural behavior: heavy alkalis Cs and Rb are mainly controlled by surfaces such as those of clay minerals; K, Na, Cl and Br stand for brines and salt minerals (halides); SO4, Ca, Sr, Mg, Ba and HCO3 represent dissolved species from carbonate-sulfate minerals (e.g., anhydrite/gypsum, aragonite and barite). All these minerals are abundant in the Dead Sea sediments. (C) Ratios between the measured ion concentrations and those calculated by a two-component mixing model (see Table 2 for the estimated mixing coefficients) using the Jericho 5 freshwater and either the Dead Sea pore water or the Qedem brine as end-members.