Abstract
Plasmacytoid dendritic cells (pDCs) have been implicated both in the control and pathogenesis of influenza virus infection. We demonstrate that pDC depletion has marked effects on the response of mononuclear phagocytes, including conventional DCs (cDCs) and macrophages, to lethal influenza virus infection. Infection of mice lacking pDCs through antibody-mediated depletion resulted in substantially increased accumulation of mononuclear phagocytes and their progenitors in lungs compared to non-treated controls. pDC ablation resulted in a 5- to 35-fold enhancement of intracellular TNF-α and IL-6 production from inflammatory cDCs and exudate macrophages. Purified pulmonary cDCs and macrophages cultured from pDC-depleted mice produced significantly elevated levels of pro-inflammatory cytokines and chemokines compared to pDC-intact counterparts. Elimination of pDCs resulted in decreased lung IFN-α and an immediate and transient reduction in lung virus burden but did not impact disease outcome. These data reveal a suppressive effect of pDCs on the inflammatory response to influenza virus infection in the lung.
Keywords: innate immunity, cellular immunity, mucosal immunity, dendritic cell, macrophage
1. Introduction
Plasmacytoid dendritic cells (pDCs) are specialized antigen presenting cells widely studied for their copious production of antiviral type I interferons (IFN) [1, 2]. However, the role of pDCs in antiviral responses, particularly at mucosal surfaces, is unclear and complex. Upon vaginal herpes simplex virus type-2 infection, pDCs are recruited to the genital lamina propria where Toll-like receptor 9-mediated IFN-α production by pDCs controls survival [3]. Similarly, pDCs appear to have a protective function in respiratory syncytial virus infection, as pDC depletion increases lung viral titers and pulmonary inflammation, whereas subsequent adoptive transfer of activated pDCs results in virus elimination from the lung [4, 5]. Conversely, in a model of asthma, pDC ablation resulted in airway sensitization upon exposure to inert antigen as pDCs were shown to inhibit conventional dendritic cell (cDC)-mediated generation of cytokine producing effector T cells [6]. Following exposure to influenza virus or human immunodeficiency virus, pDCs induce apoptosis of virally-infected and bystander cells via upregulation of the TNF-related apoptosis inducing ligand mediated by autocrine type I IFN signaling [7, 8]. Additionally, pDCs eliminate virus-specific cytotoxic lymphocytes in the lung following pathogenic influenza virus infection suggesting a pDC-mediated role in restoring immunologic homeostasis post-infection [9]. These reports suggest that pDCs may modulate the immune response to viral infection in the lung through suppression or elimination of other effector cells.
DCs and macrophages within mucosal tissues, comprising the mononuclear phagocyte system, serve at the interface between innate and adaptive immunity being continually exposed to both benign and deleterious environmental agents. Derived from a common monocyte/DC progenitor, cDCs and macrophages demonstrate vast plasticity within mucosal surfaces with overlapping functional and phenotypic properties, an effect exacerbated under inflammatory conditions [10-12]. Acknowledging this inherent heterogeneity, cDCs are considered superior in antigen transport to the draining lymph nodes and subsequent induction of naïve T cells responses, whereas macrophages predominantly capture antigen via phagocytosis [13]. Under homeostatic conditions, mononuclear phagocytes sample environmental antigens while maintaining immunologic tolerance, whereas upon viral infection these cells facilitate a coordinated innate immune response that helps establish an antiviral state and induce adaptive immunity [14].
While the majority of studies examining the antiviral role of pDCs during respiratory viral infection have focused on their impact upon adaptive immunity, little is known about the interactions between pDCs and the innate effector cells of the lung. Following intranasal infection with the highly cytotoxic mouse hepatitis virus, pDC-derived IFN-α s protection of cDCs and macrophages necessary for innate viral control [15]. Migrant airway-derived cDCs have been shown to transport and share viral antigen with resident blood-derived CD11b− CD8α+ DCs within mediastinal lymph nodes (mLNs) which subsequently cross-present viral antigens to CD8+ T cells [16]. Selective depletion of pDC during cutaneous herpes simplex virus infection impairs the ability of lymph node-resident cDCs to promote antiviral cytotoxic lymphocyte responses, an effect reversed by adoptive transfer of pDC precursors [17-19]. Yet, the influence of pDCs upon the innate cellular response during acute influenza virus infection at the lung mucosa is ill-defined.
To address this gap in our understanding we characterized the influence of pDCs upon cDC and macrophage dynamics in the lung during the course of respiratory infection with pathogenic influenza virus.
2. Materials and methods
2.1. Animal manipulations
Specific pathogen free 8-12 week female BALB/c mice obtained from Charles River Laboratories were infected intranasally with 1000 plaque forming units of influenza virus strain A/PR/8/34 (PR8) [20] in accordance with Institutional Animal Care and Use Committee guidelines of the University of marrow stromal antigen 2 (BST-2)/CD317-specific monoclonal antibody clone 120G8 (Imgenex, San Diego, CA) by intraperitoneal injection 24 hours before and at 48 hour intervals after infection [21]. For select experiments, 2 mg 5-bromo-2′-deoxyuridine (BrdU) (Sigma, St. Louis, MO) was administered in 200 μl HBSS via the intraperitoneal route 24 hours prior to sacrifice.
2.2. Tissue harvesting and sample processing
Whole lungs were collected without perfusion and were minced and incubated for 30 min in media containing collagenase and DNAse prior to cell separation [20]. Lungs were physically disrupted by passing cells through a ammonium chloride treatment. Lung samples for multiplex assays were obtained by homogenizing whole lungs in 2ml cold phosphate buffered saline and immediately freezing collected supernatant at −80°C. Absolute cell numbers per tissue were calculated by multiplying the total cell count per organ measured by hemocytometer with trypan blue exclusion of apoptotic cells by the percentage of each cell population obtained by flow cytometry.
2.3. Flow cytometry and cell sorting
Phenotypic characterization, intracellular cytokine staining and detection of BrdU incorporation were performed on fresh single cell tissue suspensions as previously described with minor modifications [22]. Autofluorescence was evaluated at 575±26nm from 488nm excitation or 450±50 from 355nm excitation following validation. All antibodies were purchased from eBiosciences (San Diego, CA) unless otherwise noted. Following incubation with antibody to mouse CD16/CD32 (clone 2.4G2) to block Fc receptors, 1-5×106 cells were labeled via 15 minute incubation with amine reactive UV Live/Dead dye (Molecular Probes, Eugene, OR) to exclude dead cells and stained for 30 minutes at 4°C with combinations of monoclonal antibodies specific for: CD3e (145-2C11), CD19 (1D3), CD49b (DX5), MHC Class II (M5/114.15.2), CD11c (N418), PDCA-1 (eBio927), and CD11b (M1/70). For intracellular cytokine detection, cells were incubated for 6 hours, the final 5 of which included 10 mg/ml brefeldin A, prior to treatment with a fixing and permeabilizing solution (Cytofix/Cytoperm, Becton Dickinson, San Jose, CA) and labeling with monoclonal antibodies to TNF-α (MP6 - XT22) and IL-6 (MP5-20F3) for 30 minutes at 4°C in the presence of permeabilizing staining buffer. In select experiments, nuclear BrdU detection was performed using a BrdU detection kit (BD BrdU Flow Kit, BD Pharmingen, San Jose, CA) as per the manufacturer’s instructions. Cell staining was analyzed on an LSR II flow cytometer (Becton Dickinson). For cell sorting experiments, up to 2.0×107 freshly isolated cells from pooled lungs were treated and stained as above for lineage markers, MHC Class II, CD11c, and PDCA-1 prior to sorting on a FACSAria cytometer (Becton Dickinson). BD FACS Diva software (version 5.0.2) was used for analysis.
2.4. Multiplex and ELISA assays
Lung homogenates were thawed and IFN-α production was detected by protein-specific ELISA following manufactures instructions (PBL Interferon Source, Piscataway, NJ) [20]. FACS-sorted MHC class II+ CD11c+ mononuclear phagocytes including both cDC and activated macrophages were cultured for 24 hour without exogenous stimulation and supernatants were subsequently analyzed by or protein multiplex for the cytokines (IL-1α, IL -1β, -2, IL-3, IL-4, IL IL-5, IL-6, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-17, IFN-γ, and -α) TNF and -CSF, GM-CSF, KC (the mouse homologous to human IL-8), CCL2, CCL3, CCL4, CCL5, and CCL11) according to the manufacturer’s instructions (Bio-Rad, Hercules, CA) [20]. Briefly, diluted capture beads were added to 96-well filter plates and washed twice prior to the addition and incubation of supernatants. All incubations were performed in the absence of light at 25°C while shaking at 300rpm. Plates then received successive antibody for 30 minutes followed by 50μl of a 1:100 dilution of Streptavidin-phycoerythrin by 50μl for 10 minutes with three washes (Bio-Plex Wash Buffer) before and after incubations. Beads were resuspended io-Plex in Assay 125μl Buffer, shaken of for B30 seconds at 1,100 rpm, and 100 beads per region were read using a Bio-Plex 200 analyzer equipped with Manager 5.0 software (Bio-Rad, Hercules, CA).
2.5. Virus quantification
Viral loads in lung tissues were determined by plaque assay [20]. Briefly, MDCK cells plated in 6-well tissue culture plates were inoculated with 0.1 ml of virus-containing sample, serially diluted in Dulbecco’s modified Eagle’s medium (DMEM). Virus was adsorbed to cells for 1 hour and wells were then overlaid with 1.6% Bacto agar (DIFCO, BD Diagnostic Systems, Palo Alto, CA) mixed 1:1 with L-15 media (Cambrex, East Rutherford, NJ) containing antibiotics and 0.6 mg/ml trypsin (Sigma, St. Louis, MO). Plates were incubated for 5 days and cells were then fixed with 70% ethanol and stained with 1% crystal violet. Cells were then washed with deionized water to visualize plaques, which were counted manually.
2.6. Statistical analysis
Comparison of animal weights between groups was done using the non-parametric Mann-Whiney U test. All other statistical analysis was performed through the use of unpaired two-tailed Student’s t-test using GraphPad Prism version 5.00 (GraphPad Software, La Jolla, CA). Data are representative of at least three independent experiments using individual or 3-5 pooled mice per group and are expressed as mean ± SEM unless otherwise noted. All P values are two-sided with significance considered to be P<0.05.
3. Results
3.1. Identification of DCs and macrophages in the lung
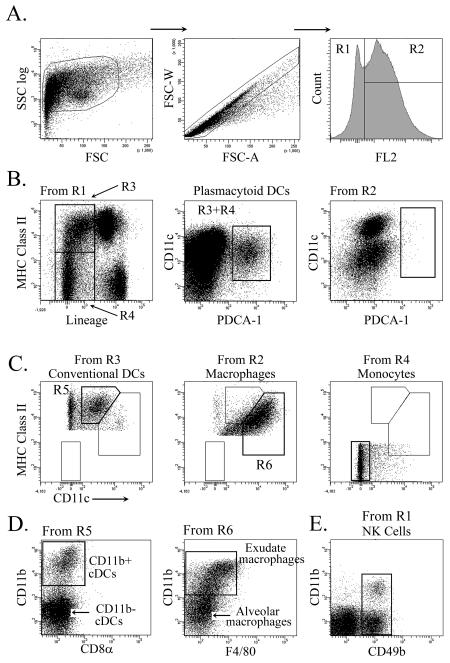
To evaluate the innate cellular immune response to influenza virus infection, mice were intranasally infected with a lethal dose of the mouse-adapted influenza virus strain PR8, which causes end-stage disease at 6-8 days after inoculation [23], precluding development of a substantial adaptive immune response. At intervals after infection lungs were harvested and the resulting cell suspensions analyzed by multiparameter flow cytometry. Progressive gating removed cellular debris and cellular aggregates (Fig.1a). Cells were treated with the amine-reactive Live/Dead viability marker to exclude dead cells. Macrophages were distinguished from DC, monocyte and NK cell populations based on high and low autofluorescence, respectively, as previously described (Fig.1a) [24]. Highly autofluorescent alveolar macrophages were identified as Lineage− (CD3, CD19, CD49b) CD11c+ cells with intermediate to high levels of MHC class II expression and lack of CD11b, while inflammation-induced exudate macrophages were further characterized by the expression of CD11b (Fig.1c, d) [25]. Present as low autofluorescent cells, monocytes lacked the expression MHC class II or CD11c (Fig.1c). Low autofluorescent pDC were defined as Lineage− MHC class II+/− CD11cInt PDCA-1+ (Fig.1b). pDCs were absent from the autofluorescent cell population, validating autofluorescence as a means of distinguishing between pDCs and macrophages (Fig. 1b). cDCs were defined as low autofluorescent Lineage− MHC class II+ CD11c+ PDCA-1− cells and further subdivided based on expression of CD11b (Fig.1c, d) [26]. NK cells were defined as autofluorescence low, CD3− CD49b+ cells with or without CD11b expression (Fig.1e) [27, 28].
Figure 1. Identification of pulmonary mononuclear phagocytes by flow cytometry.
(A) Gating strategy for lung cell suspensions based on size, doublet discrimination and low (R1) and high (R2) autofluorescence, respectively. (B) Representative dot plots illustrating gating strategy to define pDCs. Note pDCs are absent from the high autofluorescence gated population (right). (C) Representative dot plots illustrating gating to define cDCs, macrophages and monocytes. (D) Dot plots illustrating further breakdown of cDCs into CD11b+ and CD11b− subsets and macrophages into exudate and alveolar macrophages. (E) NK cells gated from R1 were identified as CD3− CD49b+ cells with or without CD11b expression.
3.2. Massive virus-induced recruitment of pulmonary cDCs and macrophages in the absence of pDCs
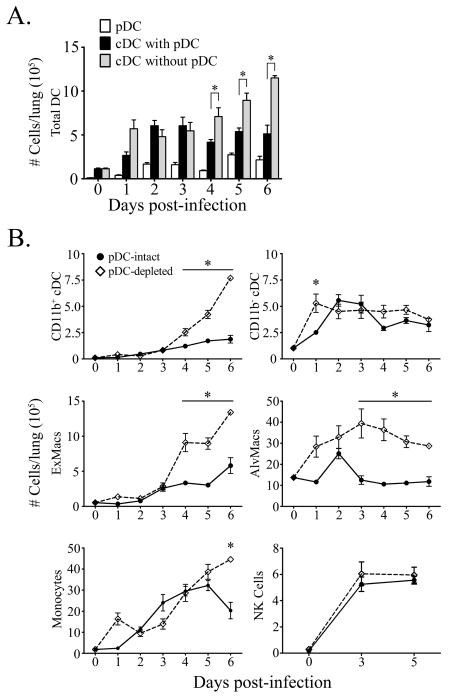
Consistent with previous reports, influenza infection of pDC-intact mice induced a 4-fold peak increase in gross cellularity in the lung within acute infection (data not shown) [20]. Significant accumulation of pulmonary cDCs, monocytes, NK cells, and alveolar and exudate macrophages was detected as early as 2 days post-infection (Fig.2a, b). Infection also induced a robust influx of pDCs into the lung, reaching peak levels of ~25 times that of naïve mice at day 5 (Fig.2a) [9, 29-32].
Figure 2. Massive recruitment of pulmonary mononuclear phagocytes during influenza virus infection in the absence of pDCs.
(A) Absolute cell counts of pDCs (white bars) and cDCs from pDC-intact (black bars) and pDC-depleted mice (grey bars) in the lung at intervals before and after influenza virus infection. (B) Absolute number of CD11b+ cDCs, CD11b− cDCs, CD11b+ exudate macrophages, CD11b− alveolar macrophages, CD11c− CD11b– monocytes, and CD49+ NK cells in the lung of pDC-intact (filled dots) and pDC-depleted mice (open diamonds). Shown are means ± SEM. * P<.05 between groups.
To determine the impact of pDCs upon the innate cellular immune response to influenza, mice were administered 120G8 antibody at 2 day intervals beginning 1 day prior to infection, which resulted in ~80-95% reduction of pDCs in the lung (data not shown), as previously described [21]. Infection of pDC-depleted mice resulted in a dramatic alteration in the mononuclear phagocyte response. In the absence of pDCs, cDCs continued to accumulate in the lung, eventually doubling in number compared to cDCs present in pDC-intact animals at day 6 post-infection (Fig.2a). Notably, pulmonary cDCs in pDC-depleted mice consisted predominantly of the CD11b+ cDC subtype, representing a greater than 4-fold increase over pDC-intact animals and 70-fold increase over homeostatic levels in naïve animals (Fig.2b). Similarly, ablation of pDCs resulted in profound and sustained enhancement of both alveolar and exudate macrophages from 3 and 4 days post-infection, respectively (Fig.2b). In contrast, accumulation of monocytes and NK cells was comparable in the lungs of pDC-intact and pDC-depleted animals throughout infection, with the exception of a late-stage divergence in the monocyte response (Fig.2b).
3.3. Enhanced cDC and macrophage generation following pDC-ablation
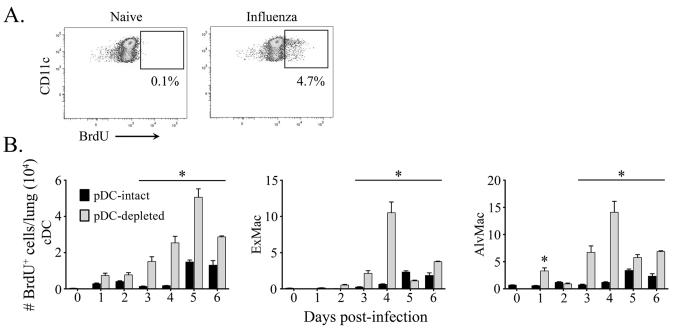
To further assess the mobilization of mononuclear phagocytes during influenza virus infection, we monitored cellular BrdU incorporation in populations of the lung 24 hours after administration [11, 33]. Consistent with bourgeoning cellularity, significantly elevated numbers of BrdU+ cDC, exudate macrophage and alveolar macrophage populations were found in the lung of pDC-depleted mice relative to pDC-intact animals (Fig.3a,b). This coincided with a marked increase in recruitment and/or proliferation of pre-DC and monocyte precursors in the lung, as measured by absolute number and in vivo BrdU incorporation in Lineage– MHC class II− CD11c+ and/or CD11b+ cells (data not shown) [11, 33]. Taken together, these findings demonstrate that pDC ablation at the time of influenza virus infection results in comparable dynamics but enhanced magnitude of production of mononuclear phagocyte progenitors and a preferential increase in recruitment of cDCs, alveolar and exudate macrophages to the lung.
Figure 3. Enhanced cDC and macrophage generation following pDC-ablation.
(A) Representative dot plots illustrating BrdU expression in cDCs prior to and following influenza virus infection. BrdU injections were given 24 hours prior to sacrifice. (B) Absolute cell counts of BrdU-positive cDCs (left), exudate macrophages (middle) and alveolar macrophages (right) from pDC-intact (black bars) and pDC-depleted mice (grey bars) in the lung at intervals following influenza virus infection. Shown are means ± SEM. * P<.05 between groups.
3.4. Increased pro-inflammatory cytokine production from lung cDCs and macrophages in pDC-depleted mice in response to influenza virus infection
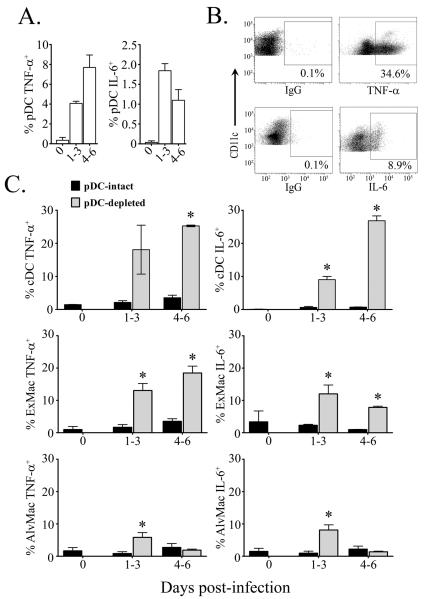
Given the marked increase in pulmonary mononuclear phagocytes in response to infection in pDC-ablated mice, we next asked whether there was a parallel enhancement in production of inflammatory cytokines by these cells. We used multiparameter flow cytometry to quantify the intracellular production of TNF-α and IL-6 ex vivo by pDCs, cDCs and macrophages in lung cell suspensions in the absence of exogenous stimulation during either early (days 1-3) or late (days 4-6) periods of infection. Infection of pDC-intact mice resulted in peak production of TNF-α and IL-6 from ~8% and 2%, respectively, of pDCs present in the lung (Fig.4a). In conjunction, concomitant TNF-α and IL-6 production was detected from cDC, exudate and alveolar macrophage populations in pDC-intact mice, albeit of a modest extent consistent with previous reports (Fig.4b, c) [34, 35]. However, infection resulted in significantly elevated cytokine responses detected in cDCs of the lung from pDC-depleted mice over those observed in pDC-intact controls. The peak frequency of cDCs from pDC-depleted mice producing TNF-α and IL-6 post-infection reached 25% and 27%, respectively, representing a 7- and 35-fold increase over levels seen in pDC-intact animals (Fig.4c). Notably, cytokine production was mediated by the CD11b+ cDC subset, representing ~80% and ~86% of TNF-α and IL-6-producing cDCs in pDC-depleted mice, respectively, compared to ~50% and ~30% TNF-α and IL-6-producing cDCs from pDC-intact controls (data not shown). Given that the absolute number of CD11b+ cDCs in lung increased 5-fold over the same period, this represents a massive increase in pro-inflammatory cytokine-producing CD11b+ cDCs in lung of influenza virus-infected mice when pDCs are absent at the time of infection.
Figure 4. Increased production of TNF-α and IL-6 from lung cDCs and macrophages in the absence of pDCs.
(A) Peak TNF-α(left) and IL-6 (right) production detected by intracellular cytokine analysis from pDCs before and during early (day 1-3) or late (day 4-6) acute influenza virus infection in pDC-intact mice. (B) Representative dot plots of TNF-αand IL-6 production from MHC class II+ CD11c+ cells post-infection. (C) Intracellular TNF-α(left) and IL-6 (right) production from cDCs (top), exudate macrophages (middle) and alveolar macrophages (bottom) from pDC-intact (black bars) and pDC-depleted mice (grey bars) before infection and during early (day 1-3) or late (day 4-6) infection. Shown are means ± SEM. * P<.05 between groups.
We next addressed antiviral cytokine production from exudate and alveolar macrophages. Within pDC-intact mice only modest production of TNF-α and IL-6 was detected from either exudate or alveolar macrophages reaching peak values of roughly 5% in either population (Fig.4c). Ablation of pDCs resulted in dramatically enhanced cytokine response from pulmonary exudate macrophages, with peaks of roughly 18% TNF-α and 12% IL-6 intracellular production detected post-infection (Fig.4c). These values represent a greater than 5-fold increase of both TNF-α IL-6 in exudate macrophages from the lungs of pDC-depleted mice compared to pDC-intact controls. Interestingly, although TNF-α and IL-6 production was augmented in alveolar macrophages from pDC-depleted mice compared to pDC-intact controls, the magnitude and duration of antiviral cytokine production was substantially reduced compared to cDC or exudate macrophage populations, with ~6% and ~8% TNF-α and IL-6, respectively, at peak post-infection (Fig.4c). Notably, by 4-6 days post-infection cytokine production from alveolar macrophages was equivalent between pDC-intact and pDC-depleted groups (Fig.4c).
3.5. Generalized increase in cytokine and chemokine responses of lung CD11c+ cells from pDC-depleted mice in response to influenza virus infection
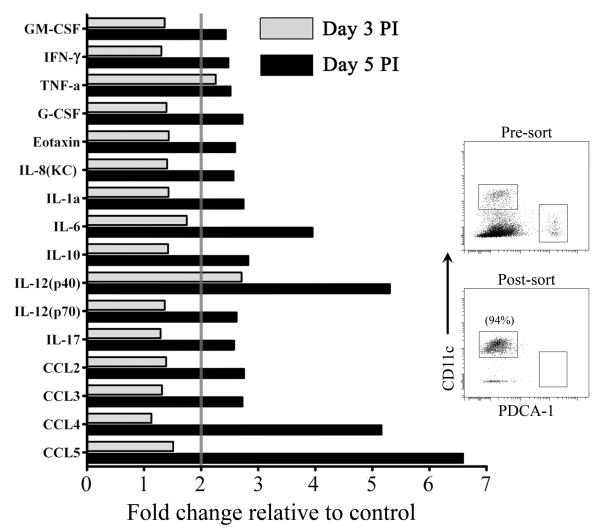
To further evaluate the impact of pDC depletion upon the innate antiviral response of pulmonary mononuclear phagocytes we purified Lineage− CD11c+ MHCII+ cells, containing both cDC and activated macrophages, from pooled lungs of normal and pDC-depleted mice harvested at 3 and 5 days post-infection and measured cytokine and chemokine levels in the supernatant by multiplex protein assay following 24 hours of culture in the absence of stimulation. By day 3 post-infection purified CD11c+ MHCII+ cells from pDC-ablated mice produced significantly more pro-inflammatory and immunoregulatory cytokines and chemokines, particularly TNF-α and IL-12p40, than pDC-intact control mice (Fig.5). By day 5 post-infection an array of pro-inflammatory and regulatory cytokines, including IFN-γ, -8, IL IL-1α, IL-6, IL-10, IL-12p70 and IL-17, were elevated to ~200% to 400% of levels found in pDC-intact mice (Fig.5). Innate production of chemokines CCL2, CCL3, CCL4 and CCL5 were increased from ~200% to 550% at day 5 post-infection in mice lacking pDCs, as was GM-CSF, a growth factor that promotes DC differentiation from monocytes and may play a role in generating recently immigrated monocyte-derived cDCs in the lung (Fig.5) [36]. Taken together, our data demonstrate that elimination of pDCs leads to robust enhancement of pro-inflammatory cytokine and chemokine production, mediated primarily by CD11b+ cDCs and exudate macrophages, during acute pathogenic influenza virus infection.
Figure 5. Generalized-activation of MHC class II+ CD11c+ cells from lungs in influenza virus infection of pDC-depleted mice.
Change in concentration of the indicated cytokines and chemokines in supernatants of purified MHC class II+ CD11c+ cells from pDC-depleted mice relative to pDC-intact mice harvested at day 3 (grey bars) and 5 (black bars) after infection and cultured for 24 hours as determined by multiplex protein assay. Data are depicted as fold change relative to pDC-intact controls. Inset illustrates pre- and post-sorted cell populations.
3.6. pDC ablation transiently reduces lung virus burden despite reduced type I IFN
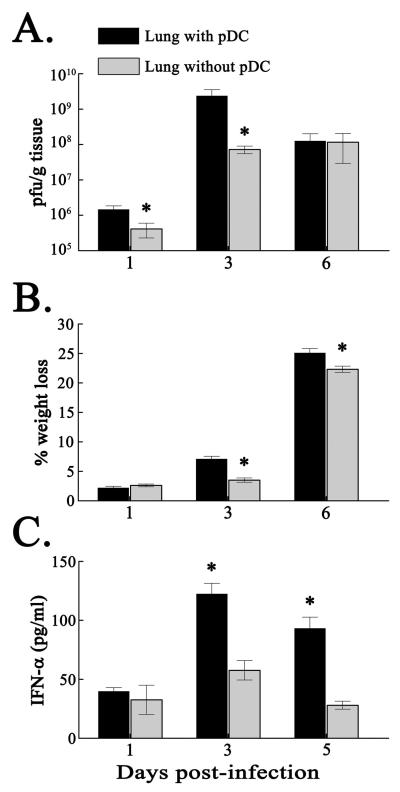
We next determined the effect of pDC ablation and the associated expansion of activated cDCs and macrophages observed in the lung on virus control and disease progression. Infection of normal mice with PR8 resulted in rapid weight loss and detection of virus in lung homogenates, consistent with previous reports [20, 37]. Interestingly, pDC-depleted animals developed modest but significantly lower viral loads within 1 day and a greater than 1 log reduction in titer at 3 days post-infection relative to influenza virus-infected mice with intact pDCs (Fig.6a). The reduction in virus titer was associated with a subsequent delay in weight loss at 3 and 6 days post-infection in pDC-depleted mice when compared to pDC-intact controls (Fig.6b). Ablation of pDCs inhibited the pulmonary IFN-α response to influenza by 50% at day 3 and 70% at day 5 post-infection consistent with a central role of pDCs in type I IFN production (Fig.6c). Nevertheless, the outcome of infection was indistinguishable between study groups with all animals being euthanized at 6 days post-infection due to progressive weight loss. These data indicate that pDC-mediated effector responses fail to aid in innate clearance of pathogenic influenza virus infection, highlighting the importance of antiviral mononuclear phagocytes in this model.
Figure 6. Depletion of pDCs transiently reduces lung virus burden and weight loss and limits lung IFN-α production.
( A) Virus titer in lung and (B) percent weight loss after influenza virus infection in pDC-intact (black bars) and pDC-depleted mice (gray bars). (C) Concentration of IFN-α in supernatants from lung pDC-intact (black bars) and pDC-depleted mice (grey bars). Shown are means ± SEM. * P<.05 between groups.
4. Discussion
In this study we analyzed the effect of pDC depletion upon the innate mononuclear phagocyte response at the lung to influenza virus infection prior to development of significant adaptive immunity by using a highly pathogenic 6-day acute infection model. We document robust recruitment, accumulation and activation of CD11b+ cDCs, exudate macrophages and alveolar macrophages in the lung in the absence of pDCs. Contrary to their role as antiviral effector cells, ablation of pDCs failed to exacerbate viral replication in the lung or accelerate disease outcome, and resulted in modest reductions of these disease parameters. Studies assessing the effect of pDCs upon adaptive immune responses to influenza virus in the pDC-deficient IkarosL/L mouse model support our findings, as Wolf et al. observed significant reductions in lung viral titers at day 4 following sublethal infection with PR8 in mice absent of pDCs compared to wild type controls without an impact on disease course [32]. In contrast to our findings, these studies showed a reduction in early pulmonary infiltrate following PR8 infection [32]. This discrepancy may be due to the effects of Ikaros depletion upon hematopoietic cells, as IkarosL/L have reduced neutrophil, B and T lymphocyte numbers and incomplete development of secondary lymphoid organs [32, 38]. These factors may limit the antiviral pulmonary response and subsequently inhibit the cytokine- and chemokine-mediated feedback loop necessary for further cellular differentiation and recruitment.
Specialized for viral detection and type I IFN production, pDCs are considered to be critical innate effector cells in initiating antiviral immunity. However, recent studies including our current findings suggest that pDCs are dispensable or even deleterious during acute influenza virus infection [9, 30, 32]. Yet the role of the pDC compartment during viral infection is biphasic, as initial efforts to inhibit viral replication through pro-inflammatory immunity give way to immunoregulatory responses aimed at restoring homeostasis. Illustrating this functional dichotomy, phenotypically distinct murine pDC subsets have been found to alternatively promote or suppress immunity with CD9+ Siglec-Hlow pDCs accountable for IFN-α cytotoxic T lymphocyte induction while CD9− Siglec-Hhigh pDCs induce Foxp3+ CD4+ T cell development [39]. Following exposure to apoptotic cells TGF-β stimulated implicated as the preeminent mediator of Treg stimulation and generation establishing an immunosuppressive environment to subvert aberrant self-directed or pathologic immunity [40]. In light of these studies, our data now suggest that pDCs exert a previously unappreciated suppressive effect on the mononuclear phagocyte response to influenza in the context of homeostatic restoration. Elimination of pDC-derived IFN-αmay alleviate immunoregulatory restrictions imposed upon mononuclear phagocytes, as the type I IFN response is implicated in secondary IL-10 production necessary for restoration of homeostasis post-infection. LPS stimulated type I IFN signaling knockout TRIFLps2/Lps2 and IRF3−/− mice develop super-induction of pro-inflammatory genes in vitro due to deficits in IL-10-dependant Stat3 phosphorylation, which mediates the anti-inflammatory response of DCs following IFN-α [41]. Similarly, mice that lack Stat3 or Toll-like receptor signaling through TRAF3 demonstrate a hyper-inflammatory DC response and reduced mucosal tolerance [42, 43]. Elimination of pDC-mediated IFN-α, facilitating the prevention of inflammatory-induced lung pathology, may therefore promote cDC and macrophage activation and recruitment during influenza virus infection.
In the current study the increase in chemokine production by activated mononuclear phagocytes in the absence of pDCs appeared to generate a positive inflammatory feedback loop resulting in amplified specific accumulation of cDCs, exudate macrophages and alveolar macrophages in the lung [25, 44]. The increased presence of phagocytic leukocytes may inhibit early viral propagation through clearance of apoptotic virally-infected cells, as the phagocytic activity of neutrophils and macrophages has been associated with reductions in influenza infected lung tissue [45]. Furthermore, studies have illustrated that administration of inhibitors of phagocytosis increased both inflammation and mortality in influenza virus infected mice [46]. In addition, pulmonary macrophage depletion via intranasal administration of clodronate-filled liposomes prior to infection with PR8 or a pathogenic 1918 recombinant H1N1 virus increased mortality and lung viral titers [47, 48]. Therefore, it is possible that removal of viral infected cells was a major contributor to viral restriction in our model. Taken together, we suggest that enhanced innate immune responses of mononuclear phagocytes following the removal of pDC-mediated suppression may compensate for the absence of pDC antiviral function.
The MHC class II+ CD11c+ CD11b+ cDC population that produced copious levels of TNF-α and IL-6 in lungs of infected mice in the absence of pDCs is consistent with an inflammatory DC that most likely differentiates from recruited Ly6C-expressing monocytes [30, 49]. A subset of DC that express high levels of Ly6C and produce TNF-α and inducible nitric oxide synthase have been defined in mice and are most frequently termed Tip-DCs (TNF/iNOS-producing DCs) [50], but have also been described as inflammatory monocytes [25, 51] and highly vacuolated foamy macrophages [44]. Tip-DCs have been shown to be important in the development of adaptive immune response in the murine lung to influenza virus infection [52]. In addition, IFN-α/β receptor-deficient mice develop a two-fold expansion of Tip-DCs and significant serum IL-12 upregulation following Listeria monocytogenes infection [53]. Recent studies utilizing genetic reporter assays have identified Tip-DCs as the major cellular source of IFN-β during Listeria monocytogenes infection [54]. Furthermore, exposure of cDCs to IFN-β either influenza infection enhanced their antiviral mRNA and cytokine responses resulting through priming INF-dependent and -independent gene regulation, resulting in impaired influenza virus replication [55]. Recent studies using parasite models indicate that MHC class II+ CD11c+ CD11b+ inflammatory DC mature into TNF-α and iNOS-producing Tip-DCs in injured tissues [56]. Whether the inflammatory cDCs identified in our study also mature into functionally distinct Tip-DCs will require further investigation.
It has been reported that BST-2, which is the target of the 120G8 depleting antibody used in our study, is upregulated on B and T lymphocytes, NK cells, cDCs and monocytes in response to type I IFN and IFN-γ [57], raising the possibility that activated innate effector cells are depleted in addition to pDCs using this treatment. This is particularly important in the potential impact of BST-2 on NK cells, which are known to play a critical role in clearance of influenza virus [58]. However, we found that NK cell recruitment to lung in response to influenza virus infection was not impaired in mice treated with BST-2, suggesting that the observed effect is not dependent upon alterations in NK cell kinetics. Furthermore, owing to the rapid lethality of this model, potential changes in T and B lymphocyte kinetics in response to antibody treatment are unlikely to influence the experimental outcome. Development of more precise targeting methods for the depletion of pDCs, such as the generation of novel pDC-specific transgenic mouse models, will further refine our understanding of the influence of pDCs upon the cDC compartment during lethal influenza virus infections [59].
It remains to be formally evaluated whether depletion of pDCs in a sublethal influenza virus infection augments the antiviral response of mononuclear phagocytes and if so whether this has an impact on disease control. However, the role of DCs in controlling influenza is likely to be complex, as Tip-DCs have been implicated in influenza-induced pulmonary immune pathology and may therefore exacerbate disease in sublethal infection [60]. It has been proposed that severe inflammation is more culpable in contributing to lung pathogenesis and fatal disease outcome during influenza infection than viral burden [25, 44, 61]. Furthermore, although innate cDC and macrophage responses may compensate for the elimination of pDC effector function during early acute infection, pDC-mediated resolution of acute inflammation and induction of an adaptive immune response is likely to be essential for homeostatic restoration [62].
Acknowledgements
The authors wish to thank Timothy Sturgeon for performing FACS sorting. This work was supported by research grant AI077771 from the US National Institutes of Health and by a grant from the Pennsylvania Department of Health.
List of Abbreviations
- pDC
plasmacytoid dendritic cell
- cDC
conventional dendritic cell
- NK cell
natural killer cell
- IFN
interferon
- IL
interleukin
- BrdU
5-bromo-2′-deoxyuridine
- PR8
A/PR/8/34 strain of influenza virus
Footnotes
Conflict of interest statement The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O’Garra A, Biron C, Briere F, Trinchieri G. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–50. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 2.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–26. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 3.Lund JM, Linehan MM, Iijima N, Iwasaki A. Cutting Edge: Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J Immunol. 2006;177:7510–4. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
- 4.Smit JJ, Rudd BD, Lukacs NW. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J Exp Med. 2006;203:1153–9. doi: 10.1084/jem.20052359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Peters N, Schwarze J. Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection. J Immunol. 2006;177:6263–70. doi: 10.4049/jimmunol.177.9.6263. [DOI] [PubMed] [Google Scholar]
- 6.de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaperot L, Blum A, Manches O, Lui G, Angel J, Molens JP, Plumas J. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J Immunol. 2006;176:248–55. doi: 10.4049/jimmunol.176.1.248. [DOI] [PubMed] [Google Scholar]
- 8.Hardy AW, Graham DR, Shearer GM, Herbeuval JP. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proc Natl Acad Sci U S A. 2007;104:17453–8. doi: 10.1073/pnas.0707244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langlois RA, Legge KL. Plasmacytoid dendritic cells enhance mortality during lethal influenza infections by eliminating virus-specific CD8 T cells. J Immunol. 2010;184:4440–6. doi: 10.4049/jimmunol.0902984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–60. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–7. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinman RM, Idoyaga J. Features of the dendritic cell lineage. Immunol Rev. 2010;234:5–17. doi: 10.1111/j.0105-2896.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- 14.Soloff AC, Barratt-Boyes SM. Enemy at the gates: dendritic cells and immunity to mucosal pathogens. Cell Res. 2010;20:872–85. doi: 10.1038/cr.2010.94. [DOI] [PubMed] [Google Scholar]
- 15.Cervantes-Barragan L, Kalinke U, Zust R, Konig M, Reizis B, Lopez-Macias C, Thiel V, Ludewig B. Type I IFN-mediated protection of macrophages and dendritic cells secures control of murine coronavirus infection. J Immunol. 2009;182:1099–106. doi: 10.4049/jimmunol.182.2.1099. [DOI] [PubMed] [Google Scholar]
- 16.Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Carbone FR, Heath WR. Cutting edge: conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- 17.Yoneyama H, Matsuno K, Toda E, Nishiwaki T, Matsuo N, Nakano A, Narumi S, Lu B, Gerard C, Ishikawa S, Matsushima K. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J Exp Med. 2005;202:425–35. doi: 10.1084/jem.20041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HK, Zamora M, Linehan MM, Iijima N, Gonzalez D, Haberman A, Iwasaki A. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J Exp Med. 2009;206:359–70. doi: 10.1084/jem.20080601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sapoznikov A, Fischer JA, Zaft T, Krauthgamer R, Dzionek A, Jung S. Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells. J Exp Med. 2007;204:1923–33. doi: 10.1084/jem.20062373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toapanta FR, Ross TM. Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res. 2009;10:112. doi: 10.1186/1465-9921-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171:6466–77. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]
- 22.Soloff AC, Liu X, Gao W, Day RD, Gambotto A, Barratt-Boyes SM. Adenovirus 5- and 35-based immunotherapy enhances the strength but not breadth or quality of immunity during chronic SIV infection. Eur J Immunol. 2009;39:2437–49. doi: 10.1002/eji.200839130. [DOI] [PubMed] [Google Scholar]
- 23.Fukushi M, Ito T, Oka T, Kitazawa T, Miyoshi-Akiyama T, Kirikae T, Yamashita M, Kudo K. Serial histopathological examination of the lungs of mice infected with influenza A virus PR8 strain. PLoS One. 2010;6:e21207. doi: 10.1371/journal.pone.0021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vermaelen K, Pauwels R. Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry A. 2004;61:170–77. doi: 10.1002/cyto.a.20064. [DOI] [PubMed] [Google Scholar]
- 25.Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180:2562–72. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 26.McGill J, Van Rooijen N, Legge KL. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med. 2008;205:1635–46. doi: 10.1084/jem.20080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, Yokoyama WM. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–8. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 28.Gazit R, Gruda R, Elboim M, Arnon TI, Katz G, Achdout H, Hanna J, Qimron U, Landau G, Greenbaum E, Zakay-Rones Z, Porgador A, Mandelboim O. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7:517–23. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 29.Ballesteros-Tato A, Leon B, Lund FE, Randall TD. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza. Nat Immunol. 2010;11:216–24. doi: 10.1038/ni.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.GeurtsvanKessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen BE, Hoogsteden HC, Osterhaus AD, Rimmelzwaan GF, Lambrecht BN. Clearance of influenza virus from the lung depends on migratory langerin+CD11b-but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–34. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18:265–77. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 32.Wolf AI, Buehler D, Hensley SE, Cavanagh LL, Wherry EJ, Kastner P, Chan S, Weninger W. Plasmacytoid dendritic cells are dispensable during primary influenza virus infection. J Immunol. 2009;182:871–9. doi: 10.4049/jimmunol.182.2.871. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Peters N, Laza-Stanca V, Nawroly N, Johnston SL, Schwarze J. Local CD11c+ MHC class II-precursors generate lung dendritic cells during respiratory viral infection, but are depleted in the process. J Immunol. 2006;177:2536–42. doi: 10.4049/jimmunol.177.4.2536. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Sesma A, Marukian S, Ebersole BJ, Kaminski D, Park MS, Yuen T, Sealfon SC, Garcia-Sastre A, Moran TM. Influenza virus evades innate and adaptive immunity via the NS1 protein. J Virol. 2006;80:6295–304. doi: 10.1128/JVI.02381-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yount JS, Kraus TA, Horvath CM, Moran TM, Lopez CB. A novel role for viral-defective interfering particles in enhancing dendritic cell maturation. J Immunol. 2006;177:4503–13. doi: 10.4049/jimmunol.177.7.4503. [DOI] [PubMed] [Google Scholar]
- 36.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O’Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–71. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 37.Tate MD, Schilter HC, Brooks AG, Reading PC. Responses of mouse airway epithelial cells and alveolar macrophages to virulent and avirulent strains of influenza A virus. Viral Immunol. 2011;24:77–88. doi: 10.1089/vim.2010.0118. [DOI] [PubMed] [Google Scholar]
- 38.Allman D, Dalod M, Asselin-Paturel C, Delale T, Robbins SH, Trinchieri G, Biron CA, Kastner P, Chan S. Ikaros is required for plasmacytoid dendritic cell differentiation. Blood. 2006;108:4025–34. doi: 10.1182/blood-2006-03-007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bjorck P, Leong HX, Engleman EG. Plasmacytoid dendritic cell dichotomy: identification of IFN-alpha producing cells as a phenotypically and functionally distinct subset. J Immunol. 2011;186:1477–85. doi: 10.4049/jimmunol.1000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonnefoy F, Perruche S, Couturier M, Sedrati A, Sun Y, Tiberghien P, Gaugler B, Saas P. Plasmacytoid Dendritic Cells Play a Major Role in Apoptotic Leukocyte-Induced Immune Modulation. J Immunol. 2011 doi: 10.4049/jimmunol.1001523. [DOI] [PubMed] [Google Scholar]
- 41.Chang EY, Guo B, Doyle SE, Cheng G. Cutting edge: involvement of the type I IFN production and signaling pathway in lipopolysaccharide-induced IL-10 production. J Immunol. 2007;178:6705–9. doi: 10.4049/jimmunol.178.11.6705. [DOI] [PubMed] [Google Scholar]
- 42.Melillo JA, Song L, Bhagat G, Blazquez AB, Plumlee CR, Lee C, Berin C, Reizis B, Schindler C. Dendritic cell (DC)-specific targeting reveals Stat3 as a negative regulator of DC function. J Immunol. 2010;184:2638–45. doi: 10.4049/jimmunol.0902960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hacker H, Redecke V, Blagoev B, Kratchmarova I, Hsu LC, Wang GG, Kamps MP, Raz E, Wagner H, Hacker G, Mann M, Karin M. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–7. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 44.Seo SU, Kwon HJ, Ko HJ, Byun YH, Seong BL, Uematsu S, Akira S, Kweon MN. Type I interferon signaling regulates Ly6C(hi) monocytes and neutrophils during acute viral pneumonia in mice. PLoS Pathog. 2011;7:e1001304. doi: 10.1371/journal.ppat.1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashimoto Y, Moki T, Takizawa T, Shiratsuchi A, Nakanishi Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J Immunol. 2007;178:2448–57. doi: 10.4049/jimmunol.178.4.2448. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe Y, Hashimoto Y, Shiratsuchi A, Takizawa T, Nakanishi Y. Augmentation of fatality of influenza in mice by inhibition of phagocytosis. Biochem Biophys Res Commun. 2005;337:881–6. doi: 10.1016/j.bbrc.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 47.Murphy EA, Davis JM, McClellan JL, Carmichael MD, Rooijen NV, Gangemi JD. Susceptibility to Infection and Inflammatory Response Following Influenza Virus (H1N1, A/PR/8/34) Challenge: Role of Macrophages. J Interferon Cytokine Res. 2011;6:501–8. doi: 10.1089/jir.2010.0143. [DOI] [PubMed] [Google Scholar]
- 48.Tumpey TM, Garcia-Sastre A, Taubenberger JK, Palese P, Swayne DE, Pantin-Jackwood MJ, Schultz-Cherry S, Solorzano A, Van Rooijen N, Katz JM, Basler CF. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79:14933–44. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–52. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 51.Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6:470–81. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aldridge JR, Jr., Moseley CE, Boltz DA, Negovetich NJ, Reynolds C, Franks J, Brown SA, Doherty PC, Webster RG, Thomas PG. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci U S A. 2009;106:5306–11. doi: 10.1073/pnas.0900655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Auerbuch V, Brockstedt DG, Meyer-Morse N, O’Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J Exp Med. 2004;200:527–33. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dresing P, Borkens S, Kocur M, Kropp S, Scheu S. A fluorescence reporter model defines “Tip-DCs” as the cellular source of interferon beta in murine listeriosis. PLoS One. 2010;5:e15567. doi: 10.1371/journal.pone.0015567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phipps-Yonas H, Seto J, Sealfon SC, Moran TM, Fernandez-Sesma A. Interferon-beta pretreatment of conventional and plasmacytoid human dendritic cells enhances their activation by influenza virus. PLoS Pathog. 2008;4:e1000193. doi: 10.1371/journal.ppat.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bosschaerts T, Guilliams M, Stijlemans B, Morias Y, Engel D, Tacke F, Herin M, De Baetselier P, Beschin A. Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-gamma and MyD88 signaling. PLoS Pathog. 2010;6:e1001045. doi: 10.1371/journal.ppat.1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–5. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 58.McGill J, Heusel JW, Legge KL. Innate immune control and regulation of influenza virus infections. J Leukoc Biol. 2009;86:803–12. doi: 10.1189/jlb.0509368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–62. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin KL, Sweeney S, Kang BD, Ramsburg E, Gunn MD. CCR2-Antagonist Prophylaxis Reduces Pulmonary Immune Pathology and Markedly Improves Survival during Influenza Infection. J Immunol. 2011;186:508–15. doi: 10.4049/jimmunol.1001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herold S, Steinmueller M, von Wulffen W, Cakarova L, Pinto R, Pleschka S, Mack M, Kuziel WA, Corazza N, Brunner T, Seeger W, Lohmeyer J. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008;205:3065–77. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matta BM, Castellaneta A, Thomson AW. Tolerogenic plasmacytoid DC. Eur J Immunol. 2010;40:2667–76. doi: 10.1002/eji.201040839. [DOI] [PMC free article] [PubMed] [Google Scholar]