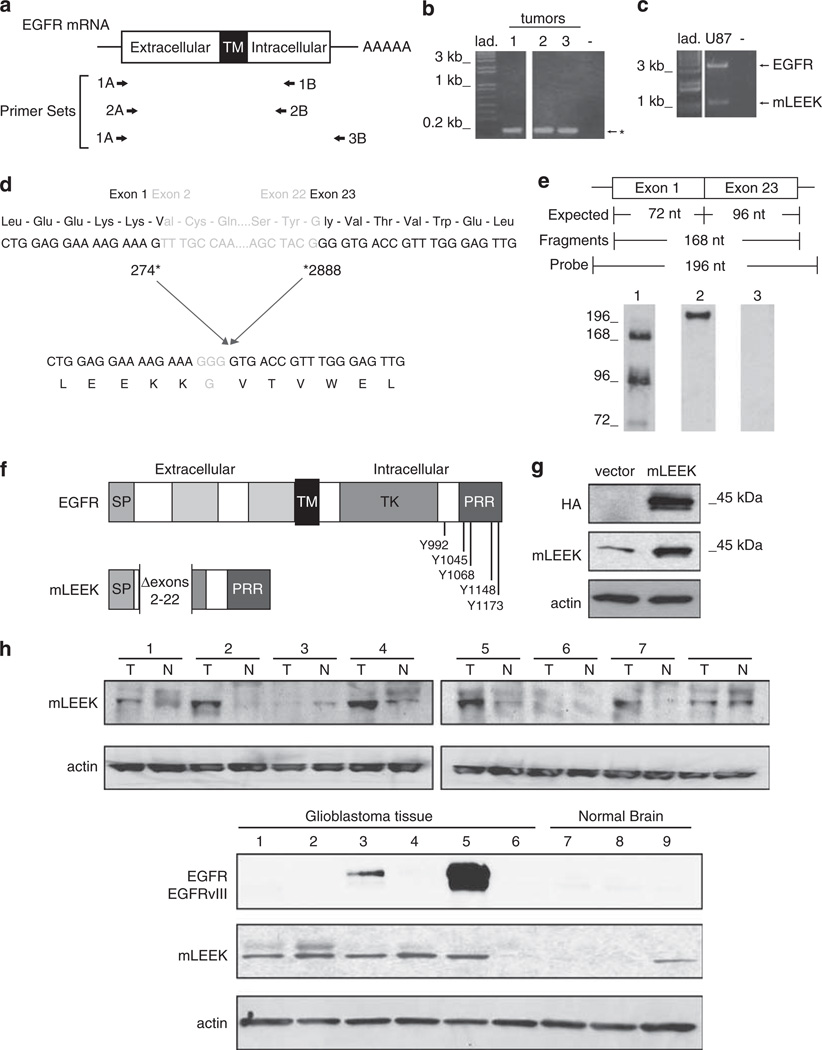
Figure 1.
mLEEK: a novel variant of the EGFR. (a) Primer sets used in RT–PCR and nested PCRs. A, sense primer; B, antisense primer. (b) RT–PCR of coding region of human EGFR using human breast tumors (1–3) and 1A/1B primers, followed by nested PCR using 2A/2B primers. Predicted sizes for full-length EGFR and EGFRvIII are 2782 and 1981 bp, respectively. *The presence of an alternative EGFR amplification product. (c) RT–PCR using 1A/3B primers and RNA from U87MG cells as template. (d) Sequencing of the alternative product identified above reveals that mLEEK is the result of the joining of exons 1–23. Numbering refers to human EGFR cDNA sequence (Accession no. X00588). (e) RNase protection detects the exon 1–23 fusion. A 196 nt. labeled antisense probe spanning the junction was used where 72 nt. are from exon 1 and 96 nucleotides come from exon 23 with an additional 28 nt. of non-complementarity to permit discrimination between full-length probe and protected fragments. The protected fragment corresponding to the junction of exons 1 and 23 is 168 nt. RNA was from A431 cells (lane 1). Undigested probe is included as a reference (lane 2). RNase protection with yeast RNA served as a negative control (lane 3). (f) Schematic of mLEEK protein structure compared to EGFR. SP, signal peptide; TM, transmembrane domain; TK, tyrosine kinase domain; PRR, proline-rich region. The known phosphorylation sites (residues numbered) are indicated. (g) Western blot of NIH3T3 cells stably expressing empty vector or mLEEK with an HA tag, probed with anti-HA, anti-mLEEK or anti-actin. (h) Top, Western blots of colon tumor lysates (T) and paired normal tissue (N), probed with anti-mLEEK antibody. Bottom, Western blot of glioblastoma tumor tissue (1–6) and normal brain tissue lysate (7–9) probed with anti-mLEEK or anti-EGFR.