Figure 1.
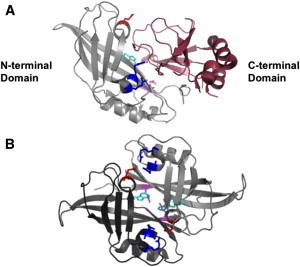
Structure of VP40 and VP40 intradimeric interface. VP40 consists of an N-terminal domain (gray) that has been shown to be involved in oligomerization and a C-terminal domain (raspberry) that has been deemed important for membrane binding. (A) VP40 x-ray structure (PDB: 1ES6) with the N-terminal domain and C-terminal domain colored in gray and raspberry, respectively. Purported residues involved in oligomerization are highlighted: Trp95 (cyan), Arg148 and Arg149 (blue), Glu160 (red), and Glu184 (magenta). (B) Antiparallel monomeric-monomeric interface (intradimeric interface with one monomer in dark gray and the second monomer in light gray) highlighting the Glu160 (red) interaction with Arg148 and Arg149 (blue), and the Trp95 (cyan) interaction with Glu184 (magenta) (PDB: 1H2D).
