Figure 4.
EB Residues Important for Growing Microtubule-End Recognition
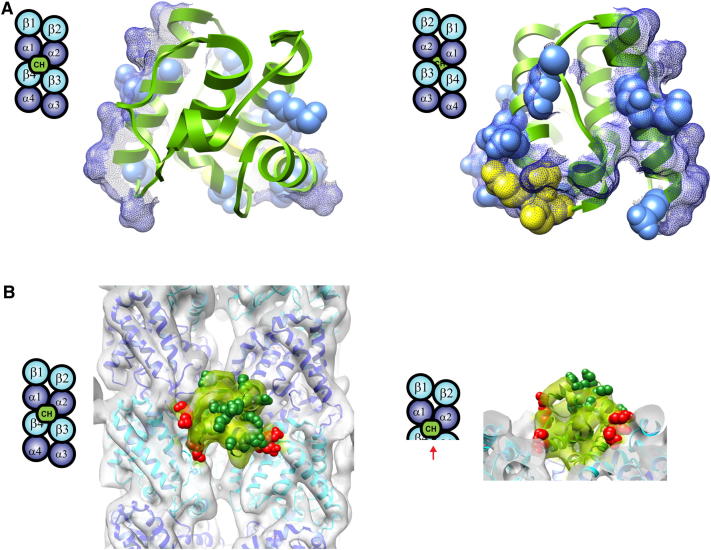
(A) Two views of conserved EB surface residues at the microtubule interface. The blue mesh depicts the surface of Mal3 CH domain residues found to be <5 Å away from tubulin residues in the pseudoatomic model. Almost all of the conserved CH domain surface residues found in the five EBs (spacefill atoms) form part of the contact surface. An especially large fraction of conserved residues (yellow spacefill) contact the β-tubulin H3 helix, whereas other conserved residues (blue spacefill) are part of the other tubulin contacts.
(B) The identified Mal3-GTPγS microtubule interface provides a structural explanation for previous mutagenesis results obtained with EB1 (Slep and Vale, 2007) (left, front view; right, end-on view from the minus end). Previous mutations shown to disrupt plus-end tracking of EB1 in cells correspond to amino acids contacting the microtubule surface (red spacefill). Our structure shows that these patches are part of contact sites between the CH domain and β3- or β4-tubulin. In contrast, mutations without a noticeable effect are distant from the microtubule surface (green spacefill).
See also Figure S3.