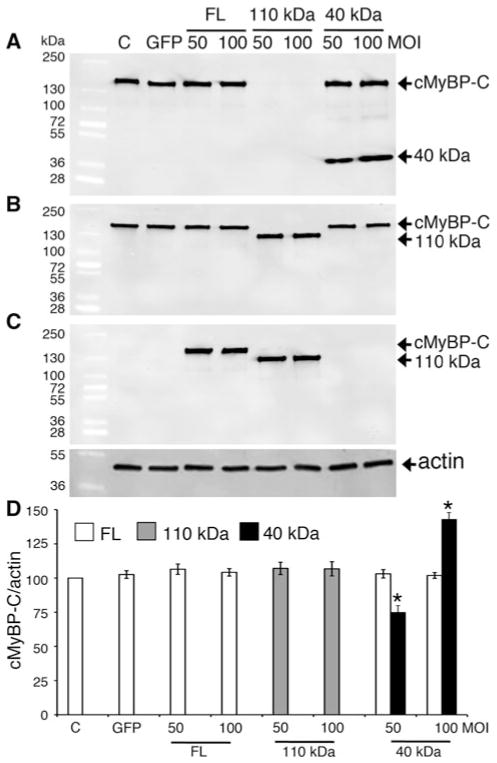
Fig. 2.
Overexpression of the 40 kDa fragments in NRVCMs does not affect expression of endogenous cMyBP-C. Representative western blot analyses show the expression of the transgenic FL, 110 and 40 kDa fragments. Fifteen micrograms of total lysates from infected NRVCMs for 48 h were used for SDS-PAGE, followed by western blot analyses with respective antibodies. FL of both endogenous and transgenic cMyBP-C and 40 kDa fragments were recognized with anti-cMyBP-C2–14 antibodies (N′-specific, (a)). FL of both endogenous and transgenic cMyBP-C and 110 kDa proteins were recognized with anti-cMyBP-CC10 antibodies (C-terminal-specific, (b)). FL and 110 kDa cMyBP-C were transgenically tagged with Myc and recognized with anti-Myc antibodies (c). Data are summarized for respective antibodies (n = 4, (d)). Sarcomeric α-actin was used as a loading control